Example 9. In an electrolysis experiment, a current was passed for 5 hours through two cells connected in series. The first cell contains a solution of gold salt and the second cell contains copper sulphate solution. 9.85 g of gold was deposited in the first cell. If the oxidation number of gold is +3, find the amount of cop- per deposited on the cathode in the second cell. Also, calculate the magnitude of the current in ampere. Solution: We know that,
Example 9. In an electrolysis experiment, a current was passed for 5 hours through two cells connected in series. The first cell contains a solution of gold salt and the second cell contains copper sulphate solution. 9.85 g of gold was deposited in the first cell. If the oxidation number of gold is +3, find the amount of cop- per deposited on the cathode in the second cell. Also, calculate the magnitude of the current in ampere. Solution: We know that,
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making...
Related questions
Question
Transcribed Image Text:Kll (Part-I)
s passed
Calculate
NTP.
passing
IL
ine)
on-
the
Electrochemistry
or
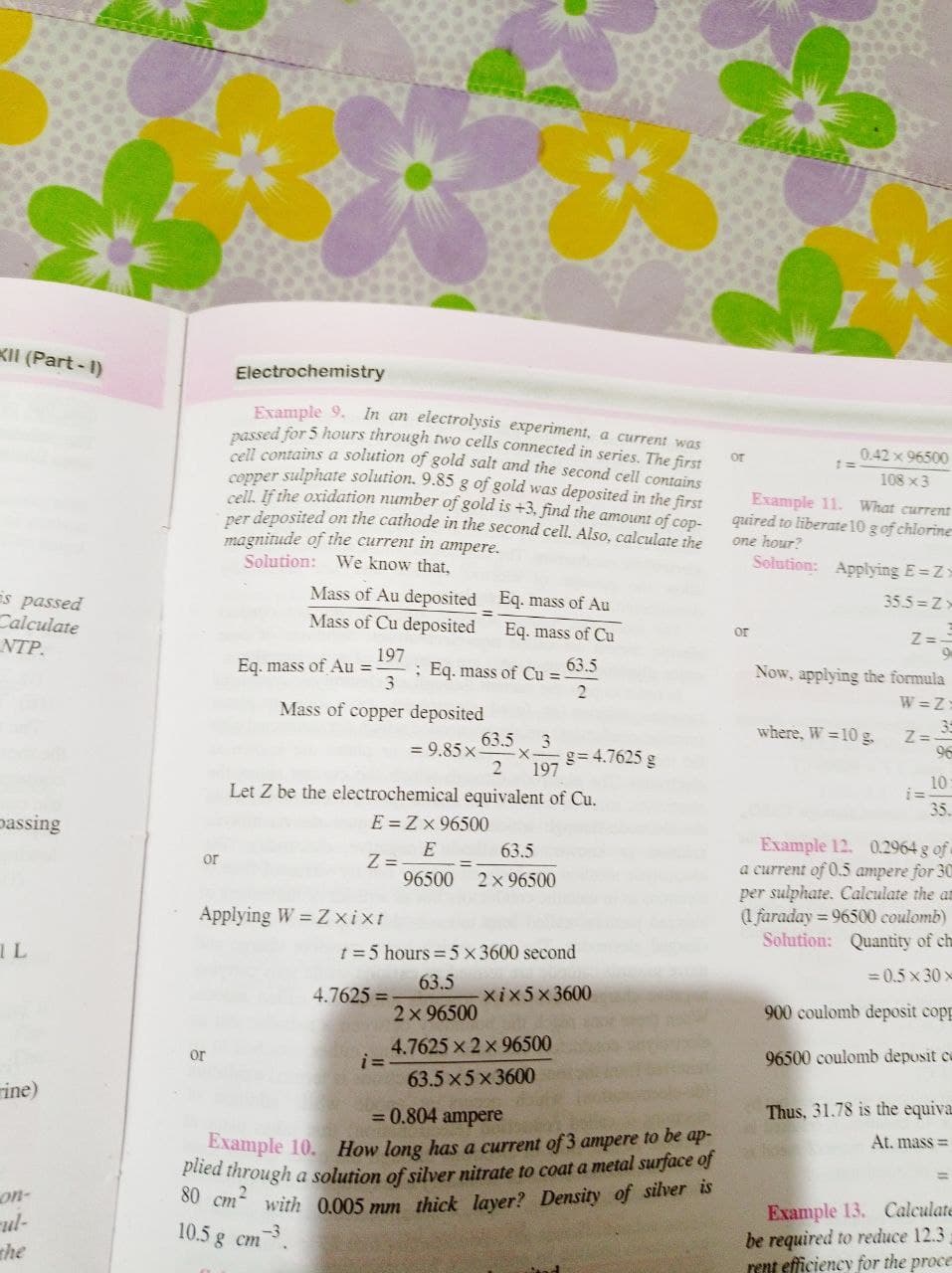
Example 9. In an electrolysis experiment, a current was
passed for 5 hours through two cells connected in series. The first
cell contains a solution of gold salt and the second cell contains
copper sulphate solution. 9.85 g of gold was deposited in the first
cell. If the oxidation number of gold is +3, find the amount of cop-
per deposited on the cathode in the second cell. Also, calculate the
magnitude of the current in ampere.
Solution: We know that,
Mass of Au deposited Eq. mass of Au
Mass of Cu deposited Eq. mass of Cu
=
Eq. mass of Au=- ; Eq. mass of Cu = 63.5
197
3
2
Mass of copper deposited
63.5
3
= 9.85 x
g= 4.7625 g
197
Let Z be the electrochemical equivalent of Cu.
E=ZX 96500
or
Z=
E
63.5
96500 2 × 96500
Applying W = Z xixt
t = 5 hours = 5 x 3600 second
4.7625=
63.5
-xix5x3600
2 × 96500
or
i=
4.7625 × 2 × 96500
63.5x5x3600
= 0.804 ampere
Example 10. How long has a current of 3 ampere to be ap-
plied through a solution of silver nitrate to coat a metal surface of
80 cm with 0.005 mm thick layer? Density of silver is
10.5 g cm
-3
0.42 × 96500
108 x 3
Example 11.
What current
quired to liberate 10 g of chlorine
one hour?
Solution: Applying E=Z=
35.5=Z-
or
Z==
Now, applying the formula
W=Z:
where, W = 10 g.
2= 35
96
10:
i=
35.
Example 12. 0.2964 g of
a current of 0.5 ampere for 30
per sulphate. Calculate the an
(1 faraday=96500 coulomb)
Solution: Quantity of ch
=0.5x30x
900 coulomb deposit copp
96500 coulomb deposit c
Thus, 31.78 is the equiva
At. mass=
Example 13. Calculate
be required to reduce 12.3
rent efficiency for the proce
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning