Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 12ALQ
Related questions
Question
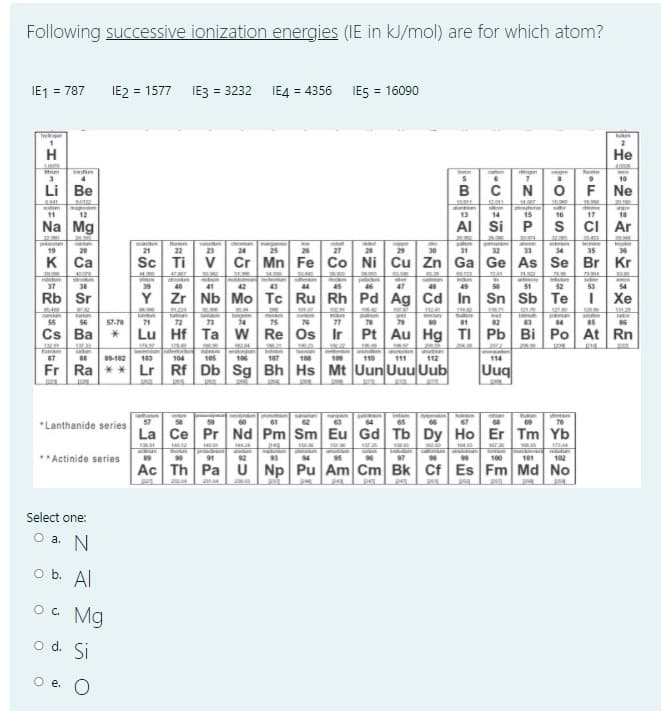
Transcribed Image Text:Following successive ionization energies (IE in kJ/mol) are for which atom?
IE1 = 787
IE2 = 1577
IE3 = 3232 IE4 = 4356 IES = 16090
H
Не
100
bomn
10
Li Be
B
F
Ne
11
12
13
14
15
16
17
18
Na Mg
AI Si
ci Ar
220
12.000
35.453
25
31
ytn
36
19
20
21
22
23
24
26
27
28
29
30
32
33
34
35
к Са
Sc
V Cr Mn Fe Co
Ni Cu Zn Ga Ge As Se Br Kr
37
38
39
40
41
42
43
44
45
46
47
48
49
51
52
53
54
Rb Sr
Y Zr Nb Mo Tc Ru Rh
Pd Ag Cd In Sn Sb Te
Xe
14
Taluy
81
56
57-70
71
72
73
75
76
77
78
79
80
82
83
84
85
86
Cs Ba
Lu Hf Taw
Re Os Ir
Pt Au Hg
TI Pb Bi Po At Rn
ann
87
183
89-102
104
105
106
107
108
109
110
111
112
114
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
*Lanthanide series
57
59
62
63
65
70
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
** Actinide series
90
91
92
95
94
95
96
97
100
101
102
Ac Th Pa
Np Pu Am Cm Bk Cf Es Fm Md No
Select one:
O a. N
o b. Al
O. Mg
o d. Si
O e. O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning