Following successive ionization energies (IE in kJ/mol) are characteristic for which one of the below atoms? IE, = 801 IE2 = 2427 IE3 = 3660 IE4 = 25026 IEs = 32827 H Не 10 Li Be B C F Ne 201 Ta m 12 otim shonstone 15 the 11 13 14 16 17 Na Mg AI Si ci Ar 21.00 pokten 19 wein 34 taram he n trnie 29 ypen 36 20 21 22 23 24 25 26 27 28 30 31 32 33 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr SIE S ntcan ton atey 37 38 39 40 41 42 43 44 45 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 91224 12129 Inl 55 56 57-70 71 72 13 14 75 76 83 4 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn IEIR 17 19-102 100 104 105 100 107 104 100 110 114 111 112 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun UuuUub Uuq nhin 57 ho n 61 62 64 06 67 70 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 1744 *Actinide series 90 91 92 93 94 95 96 97 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No p pat Select one: O a. C O b. B с. Ве
Following successive ionization energies (IE in kJ/mol) are characteristic for which one of the below atoms? IE, = 801 IE2 = 2427 IE3 = 3660 IE4 = 25026 IEs = 32827 H Не 10 Li Be B C F Ne 201 Ta m 12 otim shonstone 15 the 11 13 14 16 17 Na Mg AI Si ci Ar 21.00 pokten 19 wein 34 taram he n trnie 29 ypen 36 20 21 22 23 24 25 26 27 28 30 31 32 33 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr SIE S ntcan ton atey 37 38 39 40 41 42 43 44 45 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 91224 12129 Inl 55 56 57-70 71 72 13 14 75 76 83 4 Cs Ba Lu Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn IEIR 17 19-102 100 104 105 100 107 104 100 110 114 111 112 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun UuuUub Uuq nhin 57 ho n 61 62 64 06 67 70 *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 1744 *Actinide series 90 91 92 93 94 95 96 97 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No p pat Select one: O a. C O b. B с. Ве
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 11ALQ: Consider the following statement "The ionization energy for the potassium atom is negative, because...
Related questions
Question
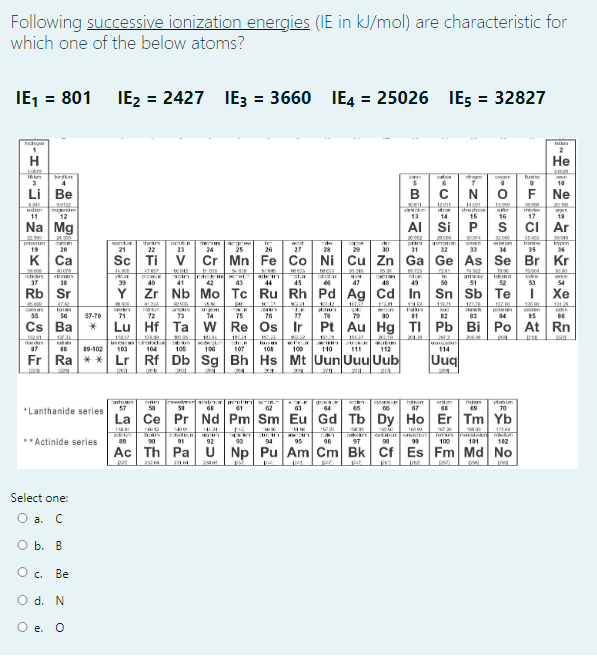
Transcribed Image Text:Following successive ionization energies (IE in kJ/mol) are characteristic for
which one of the below atoms?
IE, = 801
IE2 = 2427
IE3 = 3660 IE4 = 25026
IES
= 32827
gan
H
Не
10
Li Be
В
C N
Ne
201
KRIL
shonstor
15
th
17
otum
11
12
13
14
16
18
Na Mg
AI Si P
CI
Ar
243
pokten
19
taan
22
te n
trani
29
ypen
36
20
21
23
24
25
26
27
28
30
31
32
33
34
35
к Са
Sc Ti v Cr Mn Fe Co Ni Cu
Zn Ga Ge As Se Br Kr
SIE
ntan
37
a ey
51
54
38
39
40
41
42
43
44
45
47
48
49
52
53
Rb Sr
Y Zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te
Хе
11821
Ind
12TE
121.29
e
55
56
57-70
72
13
14
75
76
79
34
Cs Ba
Lu Hf Ta w Re Os Ir Pt Au Hg| TI Pb Bi Po At Rn
20
17
19-102
100
104
105
106
107
104
100
110
111
112
114
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
er
Tham
57
62
63
64
67
TO
*Lanthanide series
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
14
fiman
**Actinide series
96
97
89
90
91
92
93
94
95
99
100
101
102
Ac Th Pa
U Np Pu Am Cm Bk
Cf Es Fm Md No
p
pat
Select one:
O a. C
O b. B
O . Be
O d. N
O e. O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning