For a certain reaction, K. = 25.5 and k = 33.3 M.s. Calculate the value of the reverse rate constant, k, given that the reverse reaction is of the same molecularity as the forward reaction. Express your answer with the appropriate units. Include explicit multiplication within units, for example to enter M.s include (multiplication dot)
For a certain reaction, K. = 25.5 and k = 33.3 M.s. Calculate the value of the reverse rate constant, k, given that the reverse reaction is of the same molecularity as the forward reaction. Express your answer with the appropriate units. Include explicit multiplication within units, for example to enter M.s include (multiplication dot)
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
100%
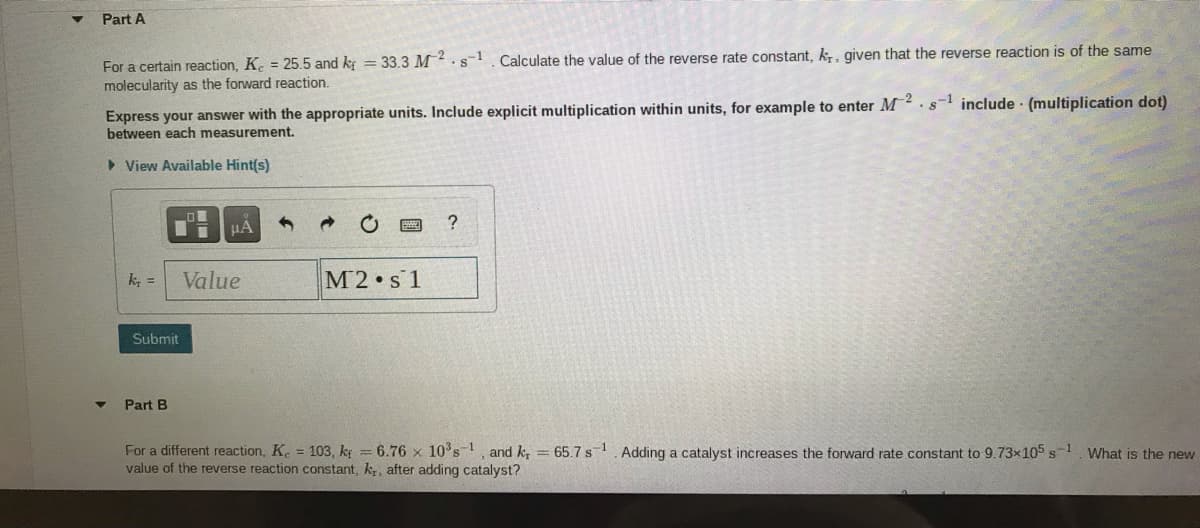
Transcribed Image Text:Part A
For a certain reaction, K. = 25.5 and kg = 33.3 M2.s1. Calculate the value of the reverse rate constant, k,, given that the reverse reaction is of the same
molecularity as the forward reaction.
Express your answer with the appropriate units. Include explicit multiplication within units, for example to enter M.s include (multiplication dot)
between each measurement.
> View Available Hint(s)
k =
Value
M2 s 1
Submit
Part B
For a different reaction, K. 103, kg = 6.76 × 10°s, and k, = 65.7 s-1. Adding a catalyst increases the forward rate constant to 9.73x105 s-1 What is the new
value of the reverse reaction constant, k, after adding catalyst?
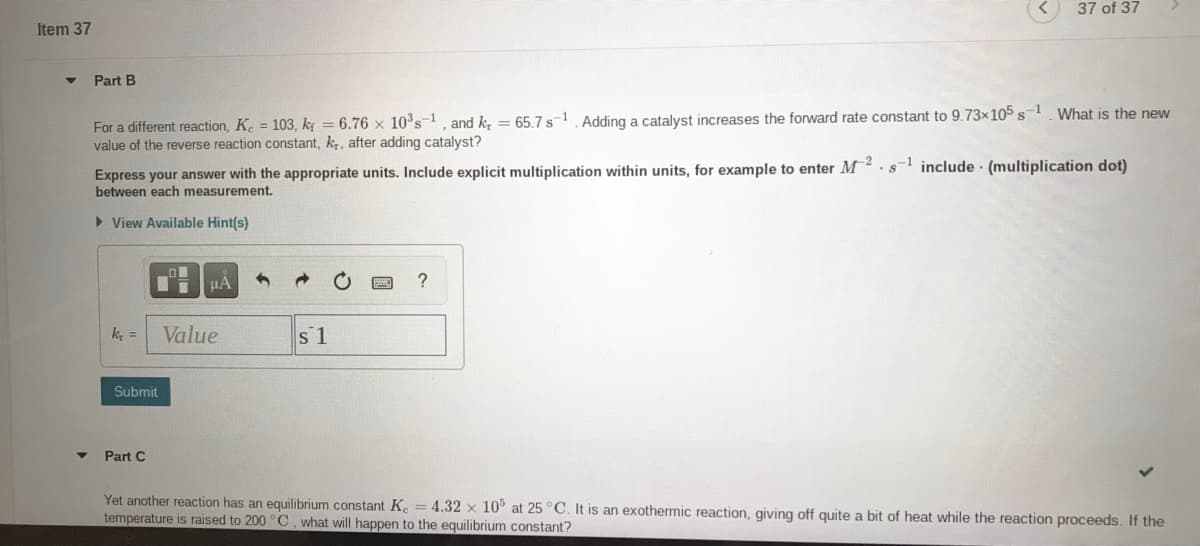
Transcribed Image Text:37 of 37
Item 37
Part B
For a different reaction, K. 103, k = 6.76 x 10°s=1, and k, = 65.7 s-1 Adding a catalyst increases the forward rate constant to 9.73x105 s1 What is the new
value of the reverse reaction constant, k, after adding catalyst?
Express your answer with the appropriate units. Include explicit multiplication within units, for example to enter M.s-1 include (multiplication dot)
between each measurement.
> View Available Hint(s)
k, =
Value
s 1
Submit
Part C
Yet another reaction has an equilibrium constant K. = 4.32 x 10° at 25 °C. It is an exothermic reaction, giving off quite a
temperature is raised to 200 °C, what will happen to the equilibrium constant?
of heat while the reaction proceeds. If the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole