For the followving reaction, 0.566 moles of sulfur dioxide are mixed with 0.423 moles of water. sulfur dioxide(g) + water(l) sulfurous acid (H, SO3)(g) What is the formula for the limiting reagent? What is the maximum amount of sulfurous acid (H2 SO3) that can be produced? moles Submit Answer Try Another Version 3 item attempts remaining
For the followving reaction, 0.566 moles of sulfur dioxide are mixed with 0.423 moles of water. sulfur dioxide(g) + water(l) sulfurous acid (H, SO3)(g) What is the formula for the limiting reagent? What is the maximum amount of sulfurous acid (H2 SO3) that can be produced? moles Submit Answer Try Another Version 3 item attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 68AP
Related questions
Question
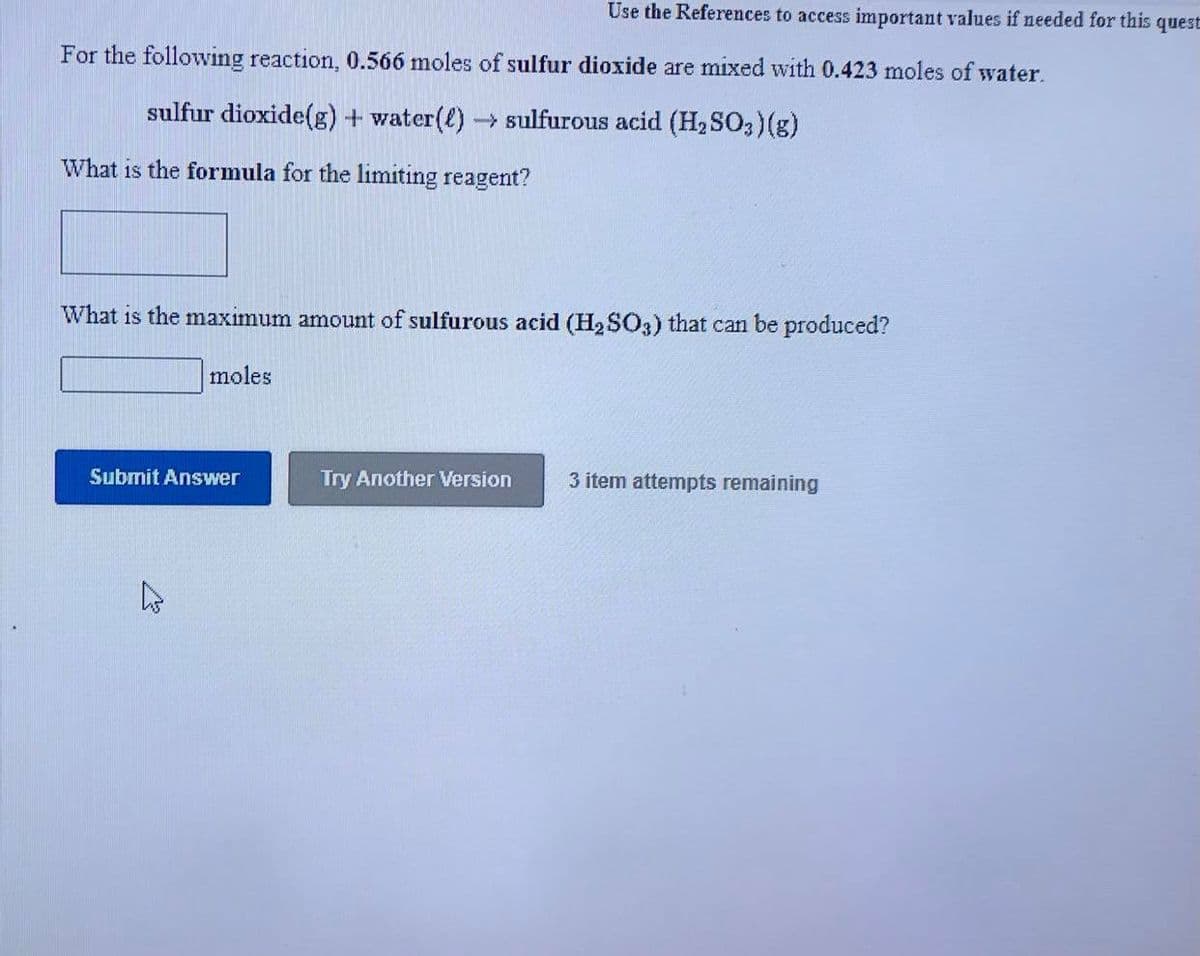
Transcribed Image Text:Use the References to access important values if needed for this quest
For the following reaction, 0.566 moles of sulfur dioxide are mixed with 0.423 moles of water.
sulfur dioxide(g) + water(l)→ Sulfurous acid (H2SO3)(g)
What is the formula for the limiting reagent?
What is the maximum amount of sulfurous acid (H2 SO3) that can be produced?
moles
Submit Answer
Try Another Version
3 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning