For the gas phase decomposition of trichloromethyl chloroformate, CICOOCCI32 COCI2 the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09×104 K and a y-intercept of 30.3. The activation energy for the gas phase decomposition of trichloromethyl chloroformate is kJ/mol.
For the gas phase decomposition of trichloromethyl chloroformate, CICOOCCI32 COCI2 the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09×104 K and a y-intercept of 30.3. The activation energy for the gas phase decomposition of trichloromethyl chloroformate is kJ/mol.
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.19QAP
Related questions
Question
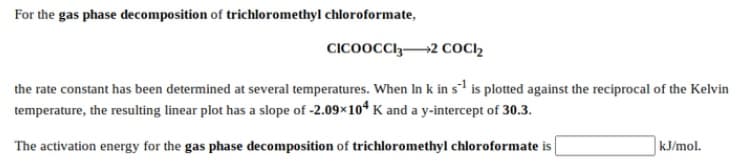
Transcribed Image Text:For the gas phase decomposition of trichloromethyl chloroformate,
CICOOCCI32 COCI2
the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin
temperature, the resulting linear plot has a slope of -2.09×104 K and a y-intercept of 30.3.
The activation energy for the gas phase decomposition of trichloromethyl chloroformate is
kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning