For the gas phase isomerization of methyl cis-cinnamate, cis-C,H;CH=CHCOOCH;→trans-CH;CH=CHCOOCH3 the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09x10ʻ K and a y-intercept of 24.3. The activation energy for the gas phase isomerization of methyl cis-cinnamate is kJ/mol.
For the gas phase isomerization of methyl cis-cinnamate, cis-C,H;CH=CHCOOCH;→trans-CH;CH=CHCOOCH3 the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting linear plot has a slope of -2.09x10ʻ K and a y-intercept of 24.3. The activation energy for the gas phase isomerization of methyl cis-cinnamate is kJ/mol.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter22: Surfaces
Section: Chapter Questions
Problem 22.44E: Are the following processes examples of homogeneous or heterogeneous catalysis? a Hydrolysis of...
Related questions
Question
I don’t know how to do this question about activation energy.
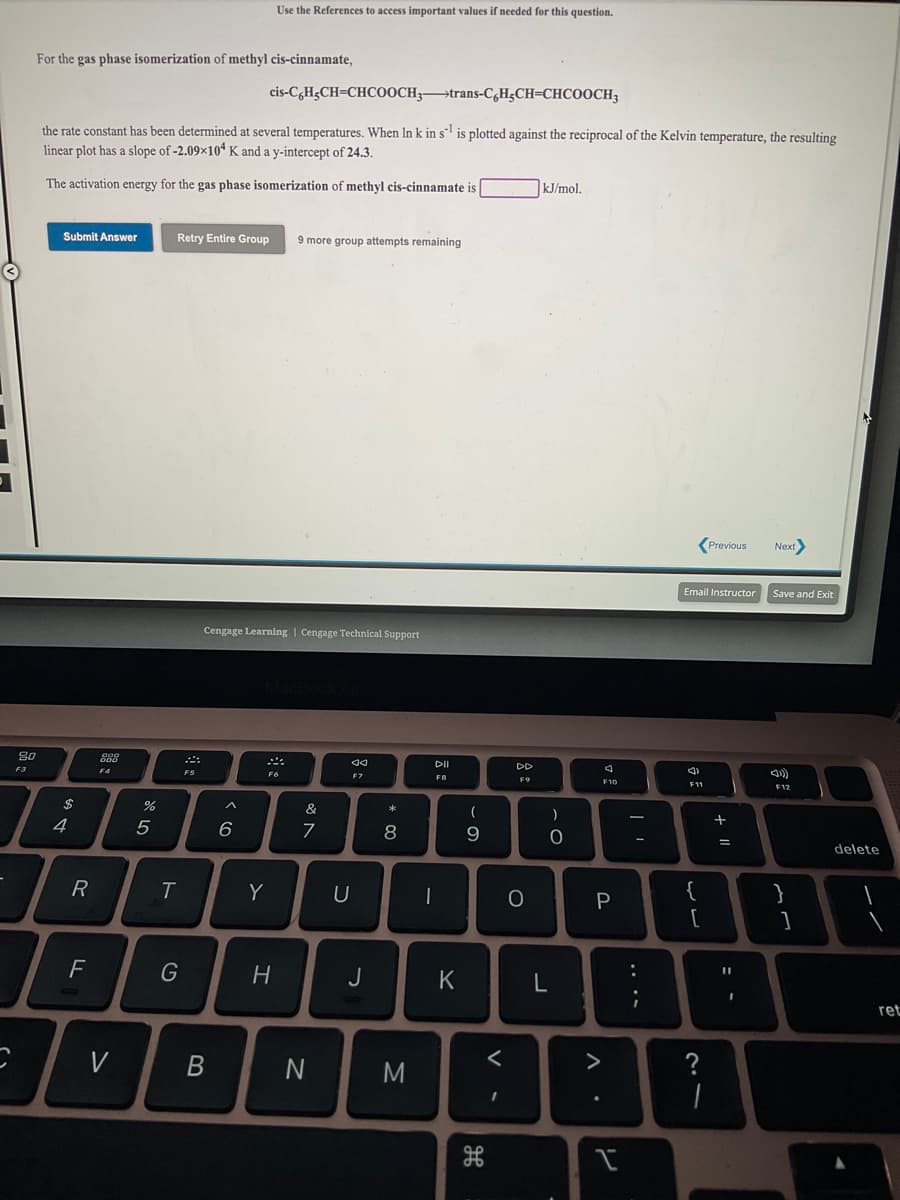
Transcribed Image Text:Use the References to access important values if needed for this question.
For the gas phase isomerization of methyl cis-cinnamate,
cis-C,H;CH=CHCOOCH3→trans-C,H;CH=CHCOOCH3
the rate constant has been determined at several temperatures. When In k in s is plotted against the reciprocal of the Kelvin temperature, the resulting
linear plot has a slope of -2.09×10“ K and a y-intercept of 24.3.
The activation energy for the gas phase isomerization of methyl cis-cinnamate is
kJ/mol.
Submit Answer
Retry Entire Group
9 more group attempts remaining
(Previous
Next)
Email Instructor
Save and Exit
Cengage Learning | Cengage Technical Support
80
888
DI
DD
F3
Fア
F11
4
5
6
8
9.
delete
R
Y
U
{
G
J
K
ret
V
M
レ
V
ト
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning