Free Energy Practice Problems Determine the Free Energy & Spontaneity for each scenario A enthalpy (kl/mol) A entropy U/K) A Free Energy (kl/mol) Ender- or exergonic? Reaction Spontaneous or not? A+B> AB +12 -5 CD > C+D -32 +25 CH:+ 202> C02+ -890 -243 2H:0 Nz+ 3H2> 2NH: -92 -199 Hydrolyzing ATP → -0.31 ADP + P. Phosphorylation of Glucose (glucose + P.) +14 20oCl: + H:0 → CO: + 2HCI *** -223 +284 *** Phosgene, COClz, was used as a weaponized gas during World War I. It reacts with moisture in the lungs to produce HCI, which causes the lungs to fill with fluid, leading to death. Use the energy values above, at a body temp of 37°C (310K) to see if this reaction is spontaneous or not.
Free Energy Practice Problems Determine the Free Energy & Spontaneity for each scenario A enthalpy (kl/mol) A entropy U/K) A Free Energy (kl/mol) Ender- or exergonic? Reaction Spontaneous or not? A+B> AB +12 -5 CD > C+D -32 +25 CH:+ 202> C02+ -890 -243 2H:0 Nz+ 3H2> 2NH: -92 -199 Hydrolyzing ATP → -0.31 ADP + P. Phosphorylation of Glucose (glucose + P.) +14 20oCl: + H:0 → CO: + 2HCI *** -223 +284 *** Phosgene, COClz, was used as a weaponized gas during World War I. It reacts with moisture in the lungs to produce HCI, which causes the lungs to fill with fluid, leading to death. Use the energy values above, at a body temp of 37°C (310K) to see if this reaction is spontaneous or not.
Chapter2: Decimal Fractions
Section: Chapter Questions
Problem 13PP
Related questions
Question
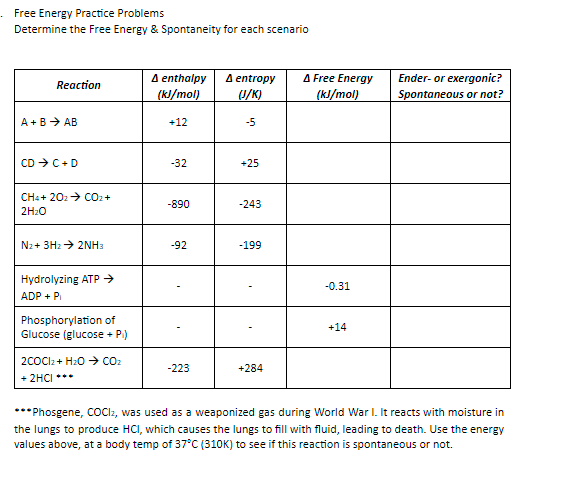
Transcribed Image Text:Free Energy Practice Problems
Determine the Free Energy & Spontaneity for each scenario
A enthalpy
(kl/mol)
A entropy
U/K)
A Free Energy
(kl/mol)
Ender- or exergonic?
Reaction
Spontaneous or not?
A +B > AB
+12
-5
CD > C+D
-32
+25
CH4+ 202> CO2+
-890
-243
2H:0
Nz+ 3H2 > 2NH3
-92
-199
Hydrolyzing ATP →
ADP + P
-0.31
Phosphorylation of
Glucose (glucose + P)
+14
2cOCl2 + Hz0 > CO2
-223
+284
+ 2HCI ***
***Phosgene, COCI2, was used as a weaponized gas during World War I. It reacts with moisture in
the lungs to produce HCI, which causes the lungs to fill with fluid, leading to death. Use the energy
values above, at a body temp of 37°C (310K) to see if this reaction is spontaneous or not.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College


Biology Today and Tomorrow without Physiology (Mi…
Biology
ISBN:
9781305117396
Author:
Cecie Starr, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College


Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning
