General Chemistry II Laboratory Manual, 2019 Revsion Questions: 39 5 When you set up the temperature probe using Capstone, you did not calibrate it to tead the correct temperature. It's possible that the probe reads temperatures that are consistently off by 1 or 2°C. Why won't this affect your results for this experiment? Explain your answer. four 2) What do you think is a source of error for this experiment? Do not answer "Errors in weighing or calculations." Sources of enron (orlld mc cold mc the foll oring. loss of sorute Through transferng, recording tempeiatines at wn the sorutron ngurou (to ensure a homugenoru i Solumon) and e Buigmg, contammamon Of T butan sufrent amnr of erren Imes, not STiring Sumple, nuA Obtainmy 3) If some t-butanol were lost between after it's weighed in before the first run, would the experimental molar mass of solute be affected? Explain your answer. how nores soute mne) xq soluent T=K( 13 lost in it sorvent werynt some heuw, ATA will mirease. This If scrvent 29 General Chemistry II Laboratory Manual, 2019 Revsion Determination of Molar Mass by Freezing Point Depression m= meres of Objectives: 1) To use freezing point depression to determine the molar mass of a solute. 2) To learn how to collect temperature data using computers. kg soluent Background: There are four colligative properties: osmotic pressure, solution vapor pressure, b ne point elevation, and freezing point depression. A colligative property is a property related to the concentration of solute particles in solution. If the solute does not dissociate in solution, colligative properties can be used to determine the molar mass of the solute. doent dissorrue.i In this lab we will use freezing point depression to determine the molar mass o an erganic compound. The change in the freezing point of a solvent is directly proportional ł (pam to the molality of the solute particles in solution. We will be using t-butanol (tertiary butanol, C4H9OH) as our solvent. The compound that we’ll use as a solute will nột dissociate easily in solution, which means that the molality of the solute will be tied directly to the number of molecules in solution, which makes it well suited for a molar mass determination. ec, m tred drrecrs to #moree ATB a m Dfp When a solvent or solution freezes, a çooling curve is observed and this can be used to 2) moral determine the freezing point of the solvent or solution. If temperature is plotted versus time when a solvent or solution is freezing, a plot such as the one shown below will an 3imw result. T but -) Sonvent od neo anoi Cooling Curve 35 34 33 32 31 30 29 28 27 tupere 26 450 400 350 300 250 200 150 100 time (s) At first there is a sharp drop in temperature. The drop in temperature becomes much less sharp when the solvent or solution begins to freeze. Sometimes the temperature will drop below the freezing point before freezing begins to occur. This is called supercooling, and has occurred in this particular plot. Temperature (deg C) 25
General Chemistry II Laboratory Manual, 2019 Revsion Questions: 39 5 When you set up the temperature probe using Capstone, you did not calibrate it to tead the correct temperature. It's possible that the probe reads temperatures that are consistently off by 1 or 2°C. Why won't this affect your results for this experiment? Explain your answer. four 2) What do you think is a source of error for this experiment? Do not answer "Errors in weighing or calculations." Sources of enron (orlld mc cold mc the foll oring. loss of sorute Through transferng, recording tempeiatines at wn the sorutron ngurou (to ensure a homugenoru i Solumon) and e Buigmg, contammamon Of T butan sufrent amnr of erren Imes, not STiring Sumple, nuA Obtainmy 3) If some t-butanol were lost between after it's weighed in before the first run, would the experimental molar mass of solute be affected? Explain your answer. how nores soute mne) xq soluent T=K( 13 lost in it sorvent werynt some heuw, ATA will mirease. This If scrvent 29 General Chemistry II Laboratory Manual, 2019 Revsion Determination of Molar Mass by Freezing Point Depression m= meres of Objectives: 1) To use freezing point depression to determine the molar mass of a solute. 2) To learn how to collect temperature data using computers. kg soluent Background: There are four colligative properties: osmotic pressure, solution vapor pressure, b ne point elevation, and freezing point depression. A colligative property is a property related to the concentration of solute particles in solution. If the solute does not dissociate in solution, colligative properties can be used to determine the molar mass of the solute. doent dissorrue.i In this lab we will use freezing point depression to determine the molar mass o an erganic compound. The change in the freezing point of a solvent is directly proportional ł (pam to the molality of the solute particles in solution. We will be using t-butanol (tertiary butanol, C4H9OH) as our solvent. The compound that we’ll use as a solute will nột dissociate easily in solution, which means that the molality of the solute will be tied directly to the number of molecules in solution, which makes it well suited for a molar mass determination. ec, m tred drrecrs to #moree ATB a m Dfp When a solvent or solution freezes, a çooling curve is observed and this can be used to 2) moral determine the freezing point of the solvent or solution. If temperature is plotted versus time when a solvent or solution is freezing, a plot such as the one shown below will an 3imw result. T but -) Sonvent od neo anoi Cooling Curve 35 34 33 32 31 30 29 28 27 tupere 26 450 400 350 300 250 200 150 100 time (s) At first there is a sharp drop in temperature. The drop in temperature becomes much less sharp when the solvent or solution begins to freeze. Sometimes the temperature will drop below the freezing point before freezing begins to occur. This is called supercooling, and has occurred in this particular plot. Temperature (deg C) 25
Chapter3: Using Spreadsheets In Analytical Chemistry
Section: Chapter Questions
Problem 3.2QAP
Related questions
Question
100%
It is an experiment on freezing point depression. I'm not sure about question q. 1

Transcribed Image Text:General Chemistry II Laboratory Manual, 2019 Revsion
Questions:
39
5 When you set up the temperature probe using Capstone, you did not calibrate it to
tead the correct temperature. It's possible that the probe reads temperatures that are
consistently off by 1 or 2°C. Why won't this affect your results for this experiment?
Explain your answer.
four
2) What do you think is a source of error for this experiment? Do not answer "Errors in
weighing or calculations." Sources of enron (orlld mc
cold mc
the foll oring. loss of sorute Through
transferng, recording tempeiatines at wn
the sorutron ngurou
(to ensure a homugenoru i Solumon) and e
Buigmg, contammamon Of T butan
sufrent amnr
of erren
Imes, not STiring
Sumple, nuA Obtainmy
3) If some t-butanol were lost between after it's weighed in before the first run,
would the experimental molar mass of solute be affected? Explain your answer.
how
nores soute
mne)
xq soluent
T=K(
13 lost in
it sorvent werynt
some heuw, ATA will mirease. This
If scrvent
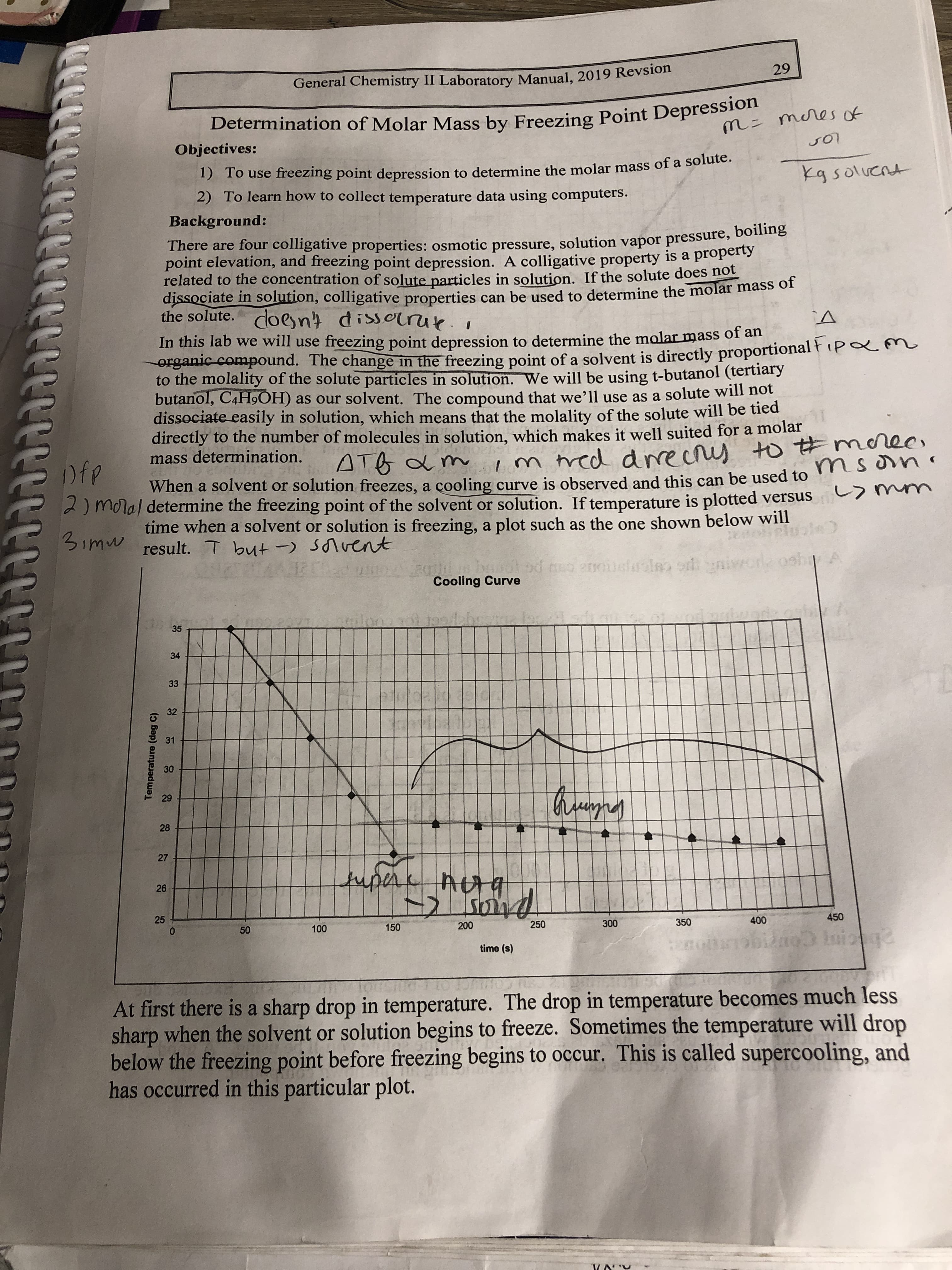
Transcribed Image Text:29
General Chemistry II Laboratory Manual, 2019 Revsion
Determination of Molar Mass by Freezing Point Depression
m= meres of
Objectives:
1) To use freezing point depression to determine the molar mass of a solute.
2) To learn how to collect temperature data using computers.
kg soluent
Background:
There are four colligative properties: osmotic pressure, solution vapor pressure, b ne
point elevation, and freezing point depression. A colligative property is a property
related to the concentration of solute particles in solution. If the solute does not
dissociate in solution, colligative properties can be used to determine the molar mass of
the solute. doent dissorrue.i
In this lab we will use freezing point depression to determine the molar mass o an
erganic compound. The change in the freezing point of a solvent is directly proportional ł (pam
to the molality of the solute particles in solution. We will be using t-butanol (tertiary
butanol, C4H9OH) as our solvent. The compound that we’ll use as a solute will nột
dissociate easily in solution, which means that the molality of the solute will be tied
directly to the number of molecules in solution, which makes it well suited for a molar
mass determination.
ec,
m tred drrecrs to #moree
ATB a m
Dfp
When a solvent or solution freezes, a çooling curve is observed and this can be used to
2) moral determine the freezing point of the solvent or solution. If temperature is plotted versus
time when a solvent or solution is freezing, a plot such as the one shown below will
an
3imw result. T but -) Sonvent
od neo anoi
Cooling Curve
35
34
33
32
31
30
29
28
27
tupere
26
450
400
350
300
250
200
150
100
time (s)
At first there is a sharp drop in temperature. The drop in temperature becomes much less
sharp when the solvent or solution begins to freeze. Sometimes the temperature will drop
below the freezing point before freezing begins to occur. This is called supercooling, and
has occurred in this particular plot.
Temperature (deg C)
25
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning



General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning