Given the following data, determine the % relative error if the true concentration of NazSO4 is 0.5000M. Express your answer in 3 decimal places. No need to include the unit. Parameter Value mass of crucible, g 43.2714 Mass of crucible + BaSO4 residue, g 46.143 Volume of sample, ml 28 Molar masses (g/mol): NazsO4 = 142.05 ; BaSO4 = 233.37 Add your answer
Given the following data, determine the % relative error if the true concentration of NazSO4 is 0.5000M. Express your answer in 3 decimal places. No need to include the unit. Parameter Value mass of crucible, g 43.2714 Mass of crucible + BaSO4 residue, g 46.143 Volume of sample, ml 28 Molar masses (g/mol): NazsO4 = 142.05 ; BaSO4 = 233.37 Add your answer
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.15QAP
Related questions
Question
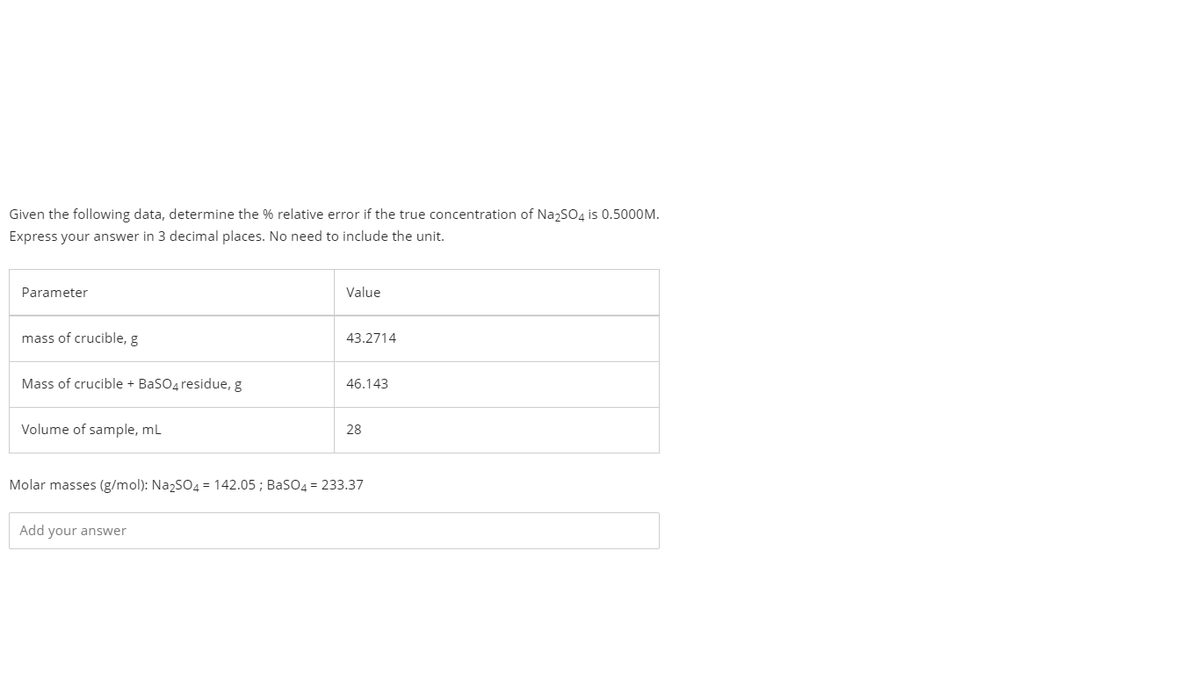
Transcribed Image Text:Given the following data, determine the % relative error if the true concentration of NazSO4 is 0.5000M.
Express your answer in 3 decimal places. No need to include the unit.
Parameter
Value
mass of crucible, g
43.2714
Mass of crucible + BaSO4 residue, g
46.143
Volume of sample, ml
28
Molar masses (g/mol): NazSO4 = 142.05 ; BasO4 = 233.37
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

