Table 4 Volume of Moles of Moles of moles of sodium acètate Log pK, pH Acetic Acid Acetic Sodium moles acetic acid mol Na Ac of Acid (mol) Acetate (mol) Solution HAC mol HAC Added (mL) 2.00 12.5 mol 0.001 mol 0.00008 4.74 4.00 6.25 mol 0.001 mol 0.00016 4.74 6.00 4.17 mol 0.001 mol 0.00024 4.74 8.00 3.13 mol 0.001 mol 0.00032 4.74
Table 4 Volume of Moles of Moles of moles of sodium acètate Log pK, pH Acetic Acid Acetic Sodium moles acetic acid mol Na Ac of Acid (mol) Acetate (mol) Solution HAC mol HAC Added (mL) 2.00 12.5 mol 0.001 mol 0.00008 4.74 4.00 6.25 mol 0.001 mol 0.00016 4.74 6.00 4.17 mol 0.001 mol 0.00024 4.74 8.00 3.13 mol 0.001 mol 0.00032 4.74
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter7: Sollutions And Colloids
Section: Chapter Questions
Problem 7.110E
Related questions
Question
100%
I need help finding the log and pH.
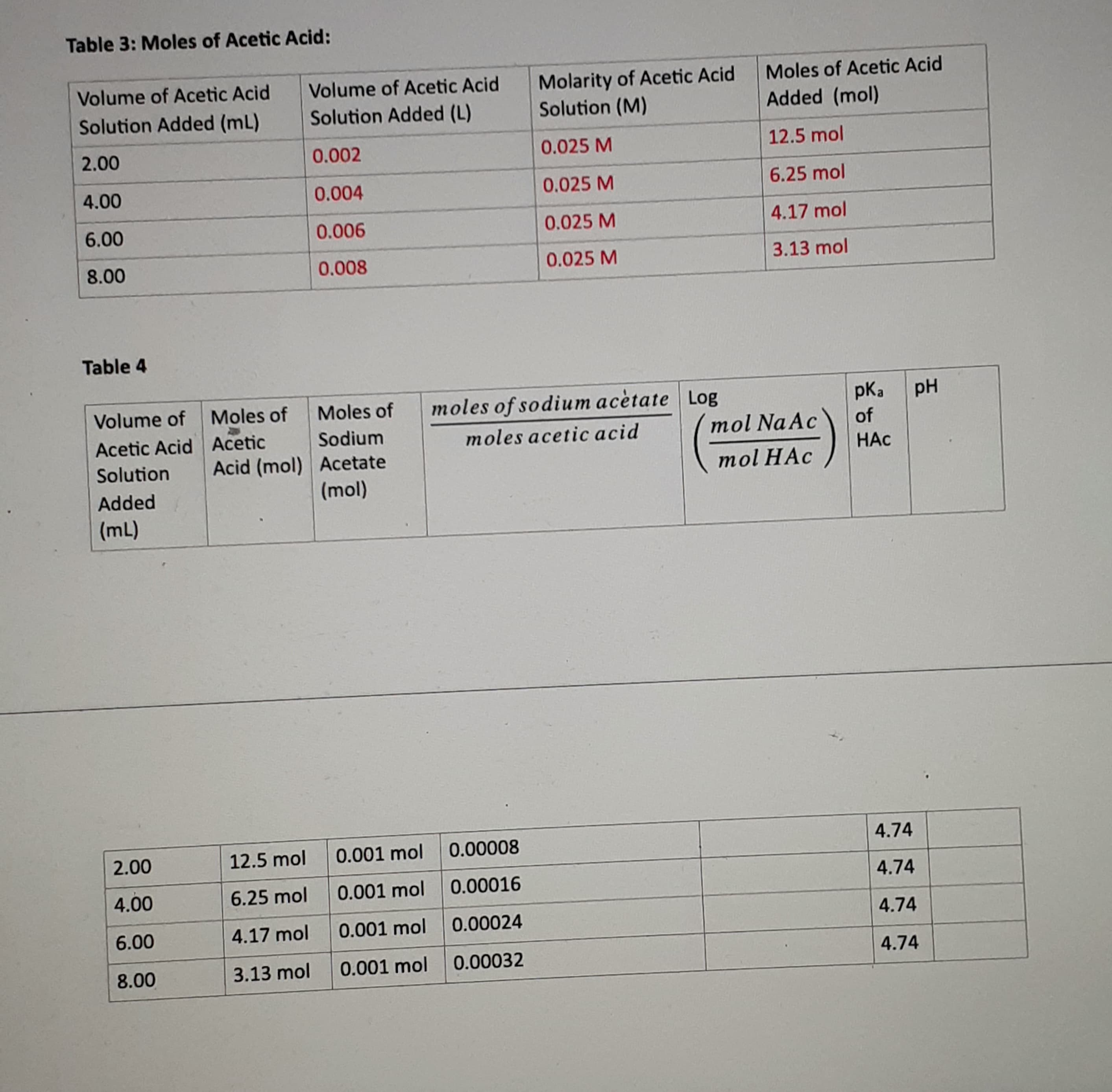
Transcribed Image Text:Table 4
Volume of
Moles of
Moles of
moles of sodium acètate Log
pK,
pH
Acetic Acid Acetic
Sodium
moles acetic acid
mol Na Ac
of
Acid (mol) Acetate
(mol)
Solution
HAC
mol HAC
Added
(mL)
2.00
12.5 mol
0.001 mol
0.00008
4.74
4.00
6.25 mol
0.001 mol
0.00016
4.74
6.00
4.17 mol
0.001 mol
0.00024
4.74
8.00
3.13 mol
0.001 mol
0.00032
4.74
Expert Solution
Step 1
Calculate the log(mol NaAc/mol HAc) value,
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning