Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter14: Acid-base Equilibria
Section: Chapter Questions
Problem 80E: Novocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization...
Related questions
Question
Hh.182.
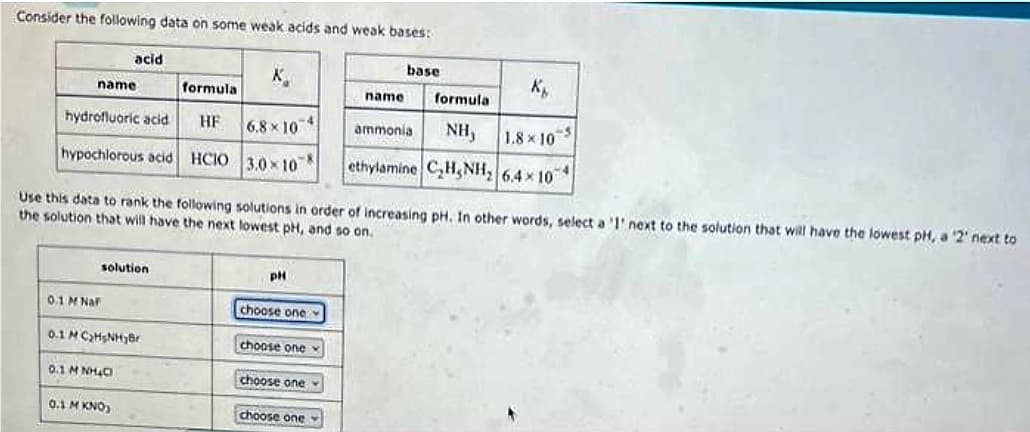
Transcribed Image Text:Consider the following data on some weak acids and weak bases:
formula
hydrofluoric acid HE
hypochlorous acid HCIO
name
acid
0.1 M Naf
solution
0.1 MC₂HsNHBr
0.1 MNH₂C
0.1 M KNO)
K
6.8x10 4
3.0x10
Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to
the solution that will have the next lowest pH, and so on.
pH
choose one
choose one
choose one
choose one
base
name
K
formula
ammonia NH, 1.8x10
0-5
ethylamine C₂H₂NH₂ 6,4 x 10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Given data
VIEWStep 2: Calculation of the pH of 0.1 M NaF solution
VIEWStep 3: Calculation of the pH of 0.1 M ethylamine hydrobromide solution
VIEWStep 4: Calculation of the pH of 0.1 M ammonium chloride solution
VIEWStep 5: Calculation of the pH of 0.1 M potassium nitrate solution
VIEWStep 6: Ranking of the given salt solutions according to their pH
VIEWSolution
VIEWStep by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning