Consider the following ion: BrO3−.
-
a) Show the full electron configuration for Br.
-
b) Draw the most correct Lewis structure for BrO3− and briefly explain why your Lewis
structure is correct.
-
c) If the structure is stabilised by resonance, draw at least one of the possible resonance
forms. If it is not stabilised by resonance, briefly explain why.
-
d) What is the electronic geometry of BrO3−? What is its molecular shape?
-
e) Does BrO3− have a dipole moment? Briefly justify your answer.
-
f) On average, would you expect IO3− to have longer or shorter bonds than BrO3−?
Briefly explain your answer.
-
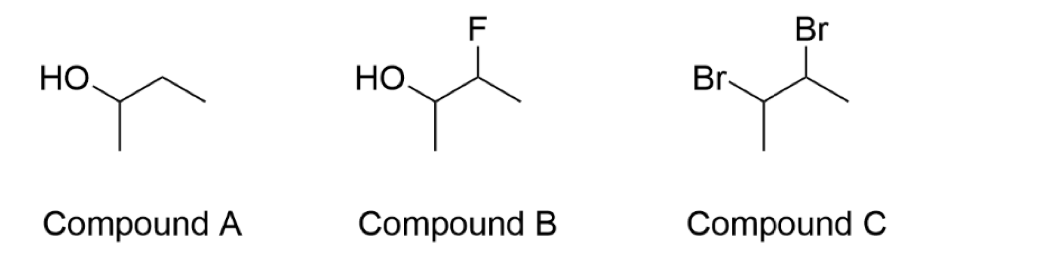
g) Which of the following molecules would you expect to have the lowest vapour
pressure? Briefly explain your choice.
-
h) What is the molecular formula for Compound C? What is the empirical formula for Compound C?
Please andwer f, g and h
the image is for g and h
Step by step
Solved in 4 steps with 4 images









