Hurricanes can travel for thousands of miles over warm water, but rapidly they lose their strength when they move over a large land mass or over cool water. Why? Explain by selecting all true statements. O As the amount of water vapor in the storm decreases, its energy decreases. Water condenses from moist air when it cools. O Moisture in the air "powers" hurricanes. As the amount of water vapor in the storm increases, its energy decreases. Water evaporates from moist air when it cools.
Hurricanes can travel for thousands of miles over warm water, but rapidly they lose their strength when they move over a large land mass or over cool water. Why? Explain by selecting all true statements. O As the amount of water vapor in the storm decreases, its energy decreases. Water condenses from moist air when it cools. O Moisture in the air "powers" hurricanes. As the amount of water vapor in the storm increases, its energy decreases. Water evaporates from moist air when it cools.
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem 4STP
Related questions
Question
100%
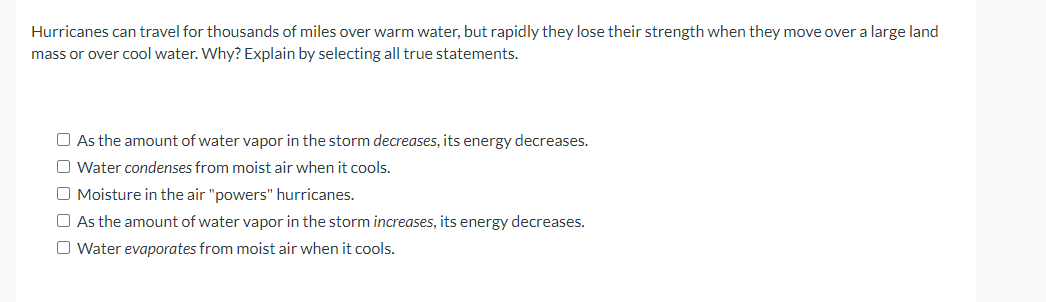
Transcribed Image Text:Hurricanes can travel for thousands of miles over warm water, but rapidly they lose their strength when they move over a large land
mass or over cool water. Why? Explain by selecting all true statements.
O As the amount of water vapor in the storm decreases, its energy decreases.
O Water condenses from moist air when it cools.
O Moisture in the air "powers" hurricanes.
O As the amount of water vapor in the storm increases, its energy decreases.
O Water evaporates from moist air when it cools.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning