i File Edit View History Window Help Bookmarks 57% Tue www-awn.aleks.com EKS Daniel Ruiz Learn YouTube Program Details Dr Pepper Tuition Giveaway mili means-Google Search mM to M O STOICHIOMETRY Solving for a reactant in solution Da One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO 4(aq) Cu(s) + FESO 4(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 146. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. x10 ? X Explanation Check
i File Edit View History Window Help Bookmarks 57% Tue www-awn.aleks.com EKS Daniel Ruiz Learn YouTube Program Details Dr Pepper Tuition Giveaway mili means-Google Search mM to M O STOICHIOMETRY Solving for a reactant in solution Da One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO 4(aq) Cu(s) + FESO 4(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 146. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. x10 ? X Explanation Check
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 44E: The flasks below illustrated three trials of a reaction between varying amounts of zinc and a...
Related questions
Question
Transcribed Image Text:i File
Edit View History
Window Help
Bookmarks
57%
Tue
www-awn.aleks.com
EKS Daniel Ruiz Learn
YouTube
Program Details Dr Pepper Tuition Giveaway
mili means-Google Search
mM to M
O STOICHIOMETRY
Solving for a reactant in solution
Da
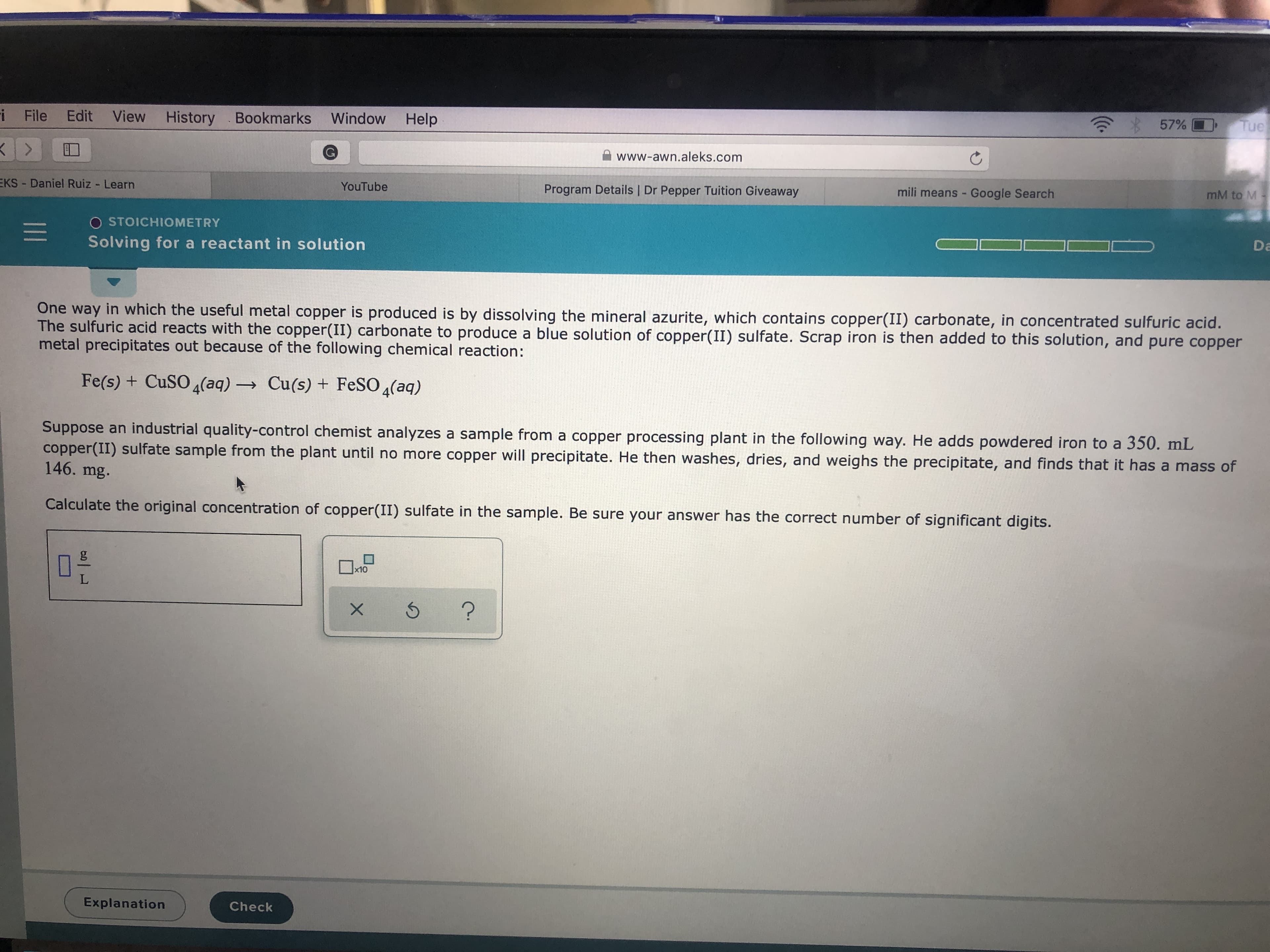
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid.
The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper
metal precipitates out because of the following chemical reaction:
Fe(s) + CuSO 4(aq) Cu(s) + FESO 4(aq)
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350. mL
copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of
146. mg.
Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.
x10
?
X
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning