I. Matching Type 1. energy at motion a. Potential energy b. Second law of 2. Energy is neither created nor destroyed 3. Work done on the system Thermodynamics c. Internal energy 4. Energy at rest 5. physical property of a material that measures how much heat is required to raise the temperature of one gram of that material by 1 K 6. Total Kinetic energy + Total Potential Energy d. +9 e. - 9 f. Specific heat capacity g. Kinetic energy 7. Heat added to the system h. First law of Thermodynamics i. - W j. k. Molar heat capacity + w
I. Matching Type 1. energy at motion a. Potential energy b. Second law of 2. Energy is neither created nor destroyed 3. Work done on the system Thermodynamics c. Internal energy 4. Energy at rest 5. physical property of a material that measures how much heat is required to raise the temperature of one gram of that material by 1 K 6. Total Kinetic energy + Total Potential Energy d. +9 e. - 9 f. Specific heat capacity g. Kinetic energy 7. Heat added to the system h. First law of Thermodynamics i. - W j. k. Molar heat capacity + w
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 11P
Related questions
Question
100%
goodmorning,, please do help me with my exercises please... because I will study this later.. thank you.
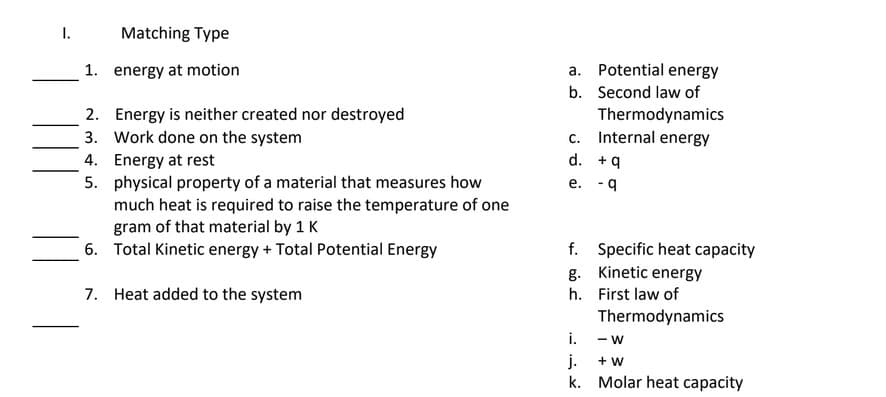
Transcribed Image Text:I.
Matching Type
1. energy at motion
a. Potential energy
b. Second law of
2. Energy is neither created nor destroyed
3. Work done on the system
4. Energy at rest
Thermodynamics
c. Internal energy
d. +9
5. physical property of a material that measures how
much heat is required to raise the temperature of one
gram of that material by 1 K
6. Total Kinetic energy + Total Potential Energy
e. - 9
f. Specific heat capacity
g. Kinetic energy
7. Heat added to the system
h. First law of
Thermodynamics
i.
- W
j.
k. Molar heat capacity
+ w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning