Icted Sample A Šample B Sample C Sample D Observations Sample E Sample F white Crstal white Fazzy Whife custallewnide (Color and appearance) Blue powda crstalClear State at Room Temperature ssold Solid sold Solid solid liquid Solubility in water Yes yes Yes Ne yes y es Color of Solution Clear clear blue clear Clear Conductivity Yes No Yes yes yes N9 Analyze: 1) Can a substance be identified as molecular or ionic based on its state at room temperature? Explain. 2) Can a substance be identified as molecular or ionic based on its solubility? Explain. 3) Can a substance be identified as molecular or ionic based on color? Explain. 4) Can a substance be identified as molecular or ionic based on conductivity of a solution? 5) What was the purpose of measuring the conductivity of distilled water before measuring the conductivity of the different solutions?
Icted Sample A Šample B Sample C Sample D Observations Sample E Sample F white Crstal white Fazzy Whife custallewnide (Color and appearance) Blue powda crstalClear State at Room Temperature ssold Solid sold Solid solid liquid Solubility in water Yes yes Yes Ne yes y es Color of Solution Clear clear blue clear Clear Conductivity Yes No Yes yes yes N9 Analyze: 1) Can a substance be identified as molecular or ionic based on its state at room temperature? Explain. 2) Can a substance be identified as molecular or ionic based on its solubility? Explain. 3) Can a substance be identified as molecular or ionic based on color? Explain. 4) Can a substance be identified as molecular or ionic based on conductivity of a solution? 5) What was the purpose of measuring the conductivity of distilled water before measuring the conductivity of the different solutions?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.14QE
Related questions
Question
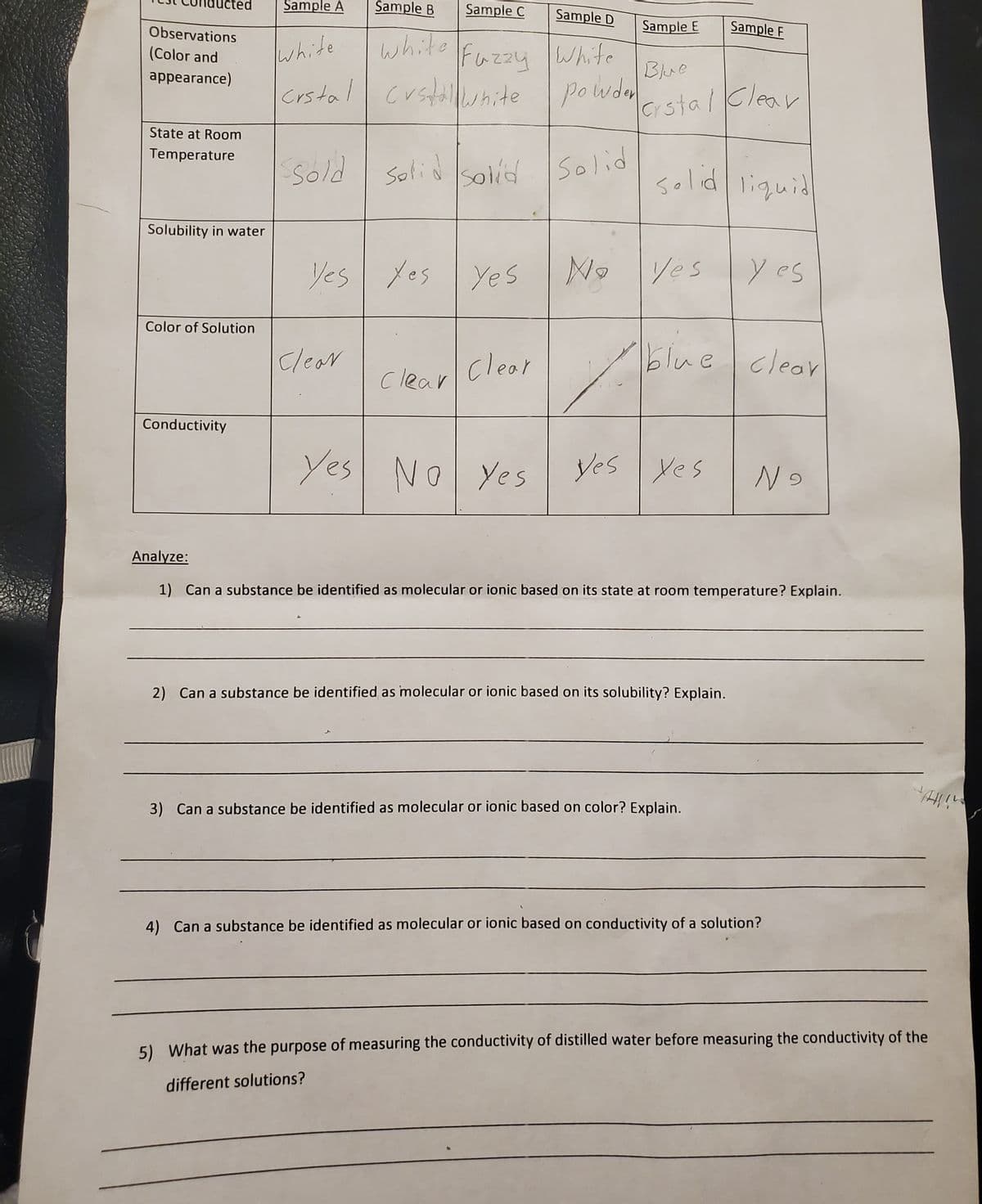
Transcribed Image Text:Sample A
Sample B
Sample C
Sample D
Observations
Sample E
Sample F
white
white
Fazzy White
(Color and
Blue
appearance)
Crstal crstalwnite
Powden
crstal Klear
State at Room
Temperature
Sold
Solid solid Solid
solid liquid
Solubility in water
Yes Yes Yes
No
yes y es
Color of Solution
clear
clear blue cleor
Clear
Conductivity
Yes No Yes
yes yes
Analyze:
1) Can a substance be identified as molecular or ionic based on its state at room temperature? Explain.
2) Can a substance be identified as molecular or ionic based on its solubility? Explain.
3) Can a substance be identified as molecular or ionic based on color? Explain.
ilth
4) Can a substance be identified as molecular or ionic based on conductivity of a solution?
5) What was the purpose of measuring the conductivity of distilled water before measuring the conductivity of the
different solutions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning