Gravimetric Analysis of a Chloride Salt Trial I Trial 2 Trial 3 0.315 8 0.969 Mass of sample 0,312g 0965 0.289 Mass of AgCl Hint:0.965-0.2893Da676 0.308.8 0959 a 287 Mass of filter paper + AgCl Mass of filter paper Mass of Cl in original sample (show calculations) a167 Hint: MCI+ AGNOS Agclis)t MNOS (NMIS the unknown metal in the original Sample mass of cl in original sample = mass of c in Agel= 0.676 x -> 35.5 1087355X100%=0.1679 %3D %3D Percent chloride in original sample (show calculations) 53. 5% Hint: C1% = 0.167g Xi00%=53.5% 0.3128 Average percent chloride (show calculations)
Gravimetric Analysis of a Chloride Salt Trial I Trial 2 Trial 3 0.315 8 0.969 Mass of sample 0,312g 0965 0.289 Mass of AgCl Hint:0.965-0.2893Da676 0.308.8 0959 a 287 Mass of filter paper + AgCl Mass of filter paper Mass of Cl in original sample (show calculations) a167 Hint: MCI+ AGNOS Agclis)t MNOS (NMIS the unknown metal in the original Sample mass of cl in original sample = mass of c in Agel= 0.676 x -> 35.5 1087355X100%=0.1679 %3D %3D Percent chloride in original sample (show calculations) 53. 5% Hint: C1% = 0.167g Xi00%=53.5% 0.3128 Average percent chloride (show calculations)
Chapter4: Least-squares And Calibration Methods
Section: Chapter Questions
Problem 7P
Related questions
Question
Im stuck on Mass of Cl in the origanal sample and average percent chloride
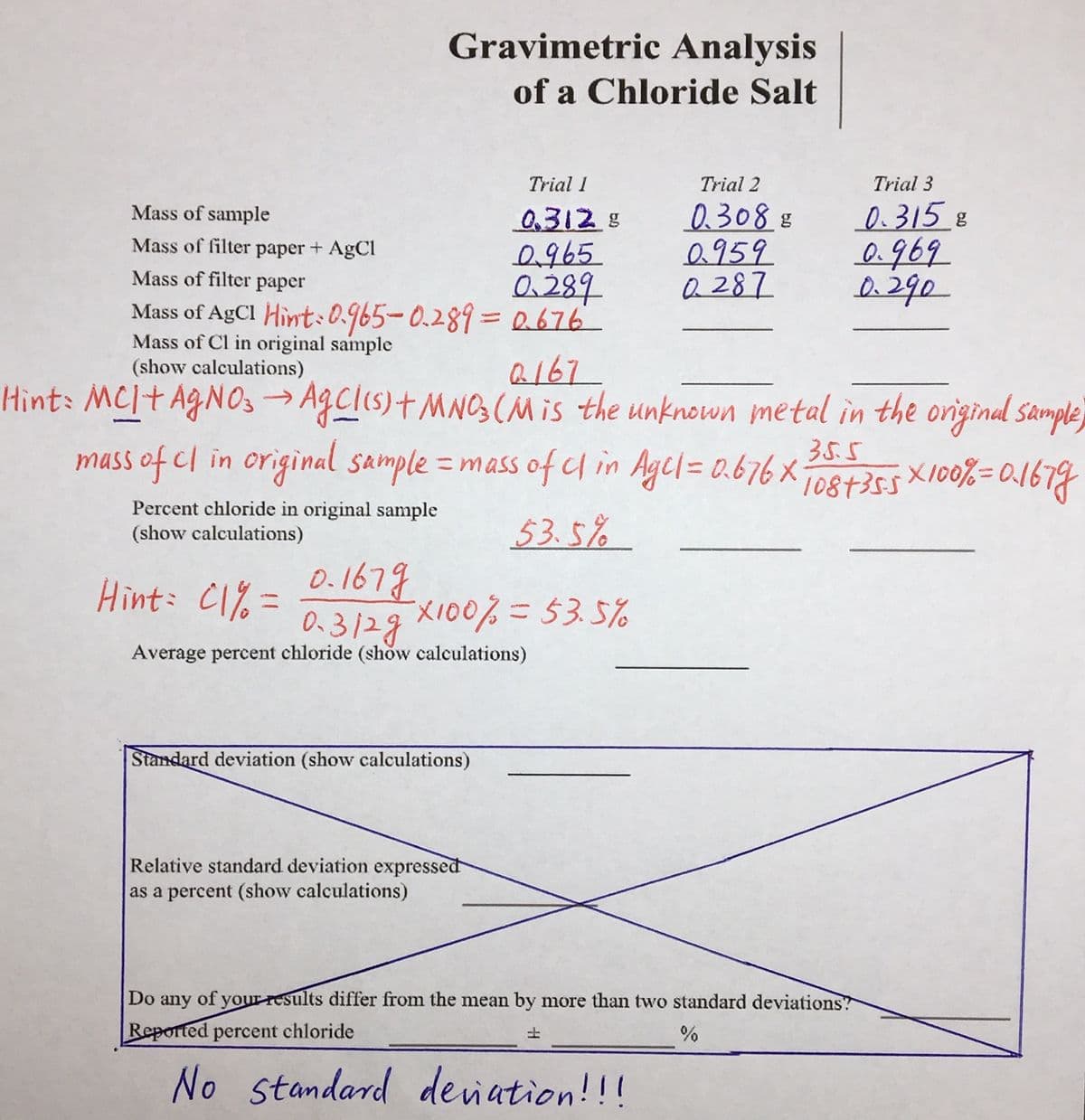
Transcribed Image Text:Gravimetric Analysis
of a Chloride Salt
Trial I
Trial 2
Trial 3
0.308 8
0959
Q 287
Mass of sample
03128
0,965
0.289
Mass of AgCl Hint:0.965-0.2893Da676
0.315 8
0.969
Mass of filter paper + AgCl
Mass of filtcer
рарer
Mass of Cl in original sample
(show calculations)
a167
Hint: MCI+ AGNO3 → AgClis)+MNO(Mis the unknown metal in the original sample
mass of cl in original sample =mass ofuin Agcl=0.676X
35.5
%3D
1087355 X100%=0.1679
Percent chloride in original sample
(show calculations)
53.5%
Hint: C1% =
0.1679
0.3128
Average percent chloride (show calculations)
Standard deviation (show calculations)
Relative standard deviation expressed
as a percent (show calculations)
Do
any
of your results differ from the mean by more than two standard deviations?
Reported percent chloride
%
土
No standard deiation!!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning