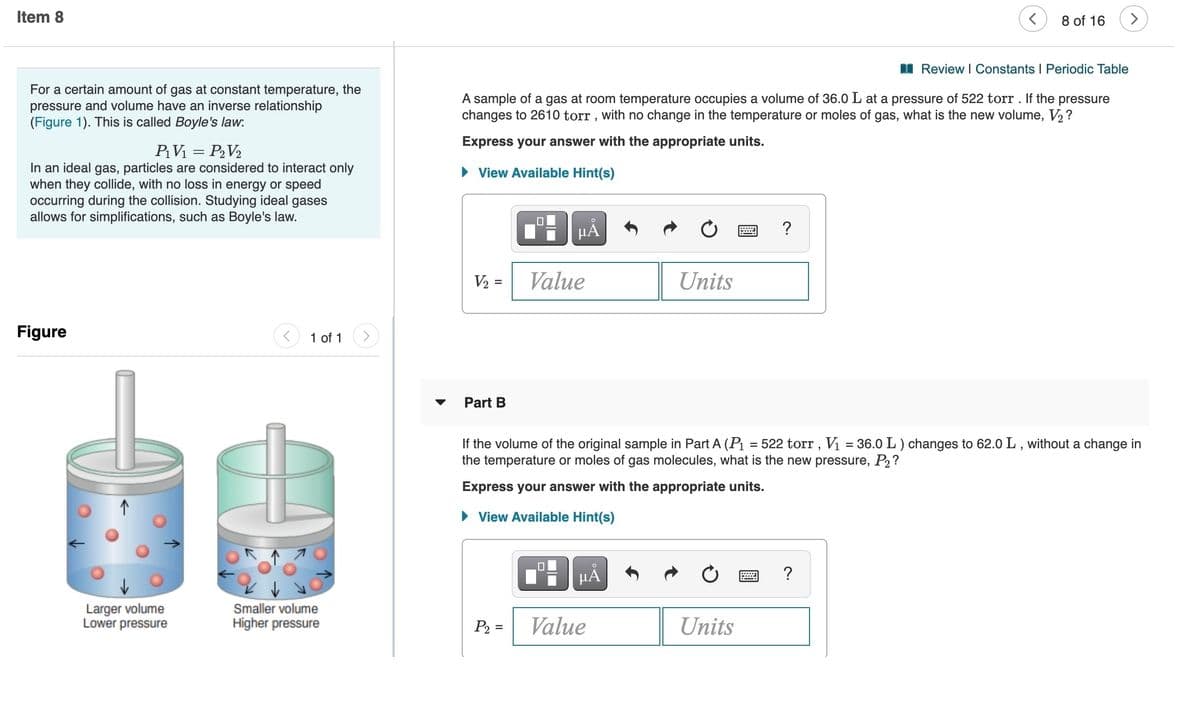
II Review i Constants i Periodic Table For a certain amount of gas at constant temperature, the pressure and volume have an inverse relationship (Figure 1). This is called Boyle's law. A sample of a gas at room temperature occupies a volume of 36.0 L at a pressure of 522 torr. If the pressure changes to 2610 torr, with no change in the temperature or moles of gas, what is the new volume, V2? Express your answer with the appropriate units. PV = PV2 In an ideal gas, particles are considered to interact only when they collide, with no loss in energy or speed occurring during the collision. Studying ideal gases allows for simplifications, such as Boyle's law. > View Available Hint(s) V2 = Value Units Figure 1 of 1 Part B If the volume of the original sample in Part A (P = 522 torr, V = 36.0 L) changes to 62.0 L, without a change in the temperature or moles of gas molecules, what is the new pressure, P2? Express your answer with the appropriate units. > View Available Hint(s) HA Larger volume Lower pressure Smaller volume Higher pressure Value P2 = Units
II Review i Constants i Periodic Table For a certain amount of gas at constant temperature, the pressure and volume have an inverse relationship (Figure 1). This is called Boyle's law. A sample of a gas at room temperature occupies a volume of 36.0 L at a pressure of 522 torr. If the pressure changes to 2610 torr, with no change in the temperature or moles of gas, what is the new volume, V2? Express your answer with the appropriate units. PV = PV2 In an ideal gas, particles are considered to interact only when they collide, with no loss in energy or speed occurring during the collision. Studying ideal gases allows for simplifications, such as Boyle's law. > View Available Hint(s) V2 = Value Units Figure 1 of 1 Part B If the volume of the original sample in Part A (P = 522 torr, V = 36.0 L) changes to 62.0 L, without a change in the temperature or moles of gas molecules, what is the new pressure, P2? Express your answer with the appropriate units. > View Available Hint(s) HA Larger volume Lower pressure Smaller volume Higher pressure Value P2 = Units
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.111P: 5-111 Diving, particularly SCUBA (Self-Contained Underwater Breathing Apparatus) diving, subjects...
Related questions
Question
Transcribed Image Text:Item 8
8 of 16
>
I Review I Constants I Periodic Table
For a certain amount of gas at constant temperature, the
pressure and volume have an inverse relationship
(Figure 1). This is called Boyle's law:
A sample of a gas at room temperature occupies a volume of 36.0 L at a pressure of 522 torr . If the pressure
changes to 2610 torr , with no change in the temperature or moles of gas, what is the new volume, V2 ?
Express your answer with the appropriate units.
PV = P2V2
In an ideal gas, particles are considered to interact only
when they collide, with no loss in energy or speed
occurring during the collision. Studying ideal gases
allows for simplifications, such as Boyle's law.
• View Available Hint(s)
HÀ
?
V2 =
Value
Units
%3D
Figure
1 of 1
Part B
If the volume of the original sample in Part A (P1 = 522 torr , V1 = 36.0 L) changes to 62.0 L , without a change in
the temperature or moles of gas molecules, what is the new pressure, P2?
%3D
Express your answer with the appropriate units.
• View Available Hint(s)
HẢ
?
Larger volume
Lower pressure
Smaller volume
Higher pressure
Value
Units
P2 =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning