In a hydrocarbon solution, the gold compound (CH3)3AUPH3 decomposes into ethane (C2H6) and a different gold compound, (CH3)AUPH3. The following mechanism has been proposed for the decomposition of (CH3)3AUPH3: Step 1: (CH3)3 AuPH; (CH3);Au + PH3 (fast) Step 2: (CH3)3 Au 4, C,H, + (CH;)Au (slow) Step 3: (CH3)Au + PH3 (CH3)AUPH3 (fast) (a) What is the overall reaction? (b) What are the inter- mediates in the mechanism? (c) What is the molecular- ity of each of the elementary steps? (d) What is the rate- determining step? (e) What is the rate law predicted by this mechanism? (f) What would be the effect on the reac- tion rate of adding PH3 to the solution of (CH3)3AUPH3?
In a hydrocarbon solution, the gold compound (CH3)3AUPH3 decomposes into ethane (C2H6) and a different gold compound, (CH3)AUPH3. The following mechanism has been proposed for the decomposition of (CH3)3AUPH3: Step 1: (CH3)3 AuPH; (CH3);Au + PH3 (fast) Step 2: (CH3)3 Au 4, C,H, + (CH;)Au (slow) Step 3: (CH3)Au + PH3 (CH3)AUPH3 (fast) (a) What is the overall reaction? (b) What are the inter- mediates in the mechanism? (c) What is the molecular- ity of each of the elementary steps? (d) What is the rate- determining step? (e) What is the rate law predicted by this mechanism? (f) What would be the effect on the reac- tion rate of adding PH3 to the solution of (CH3)3AUPH3?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.43P: Following is an equation for iodination of toluene. This reaction does not take place. All that...
Related questions
Question
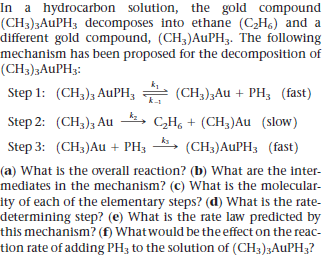
Transcribed Image Text:In a hydrocarbon solution, the gold compound
(CH3)3AUPH3 decomposes into ethane (C2H6) and a
different gold compound, (CH3)AUPH3. The following
mechanism has been proposed for the decomposition of
(CH3)3AUPH3:
Step 1: (CH3)3 AuPH; (CH3);Au + PH3 (fast)
Step 2: (CH3)3 Au 4,
C,H, + (CH;)Au (slow)
Step 3: (CH3)Au + PH3
(CH3)AUPH3 (fast)
(a) What is the overall reaction? (b) What are the inter-
mediates in the mechanism? (c) What is the molecular-
ity of each of the elementary steps? (d) What is the rate-
determining step? (e) What is the rate law predicted by
this mechanism? (f) What would be the effect on the reac-
tion rate of adding PH3 to the solution of (CH3)3AUPH3?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning