In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions), which affects lattice energy (a measure of the force needed to pull ions apart). The lattice energy affects the enthalpy of solution, which can affect solubility. Based on ion sizes, rank these compounds by their expected solubilities in water. Most soluble Least soluble Answer Bank CsBr CsI CsF CsCl
In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions), which affects lattice energy (a measure of the force needed to pull ions apart). The lattice energy affects the enthalpy of solution, which can affect solubility. Based on ion sizes, rank these compounds by their expected solubilities in water. Most soluble Least soluble Answer Bank CsBr CsI CsF CsCl
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 41A
Related questions
Question
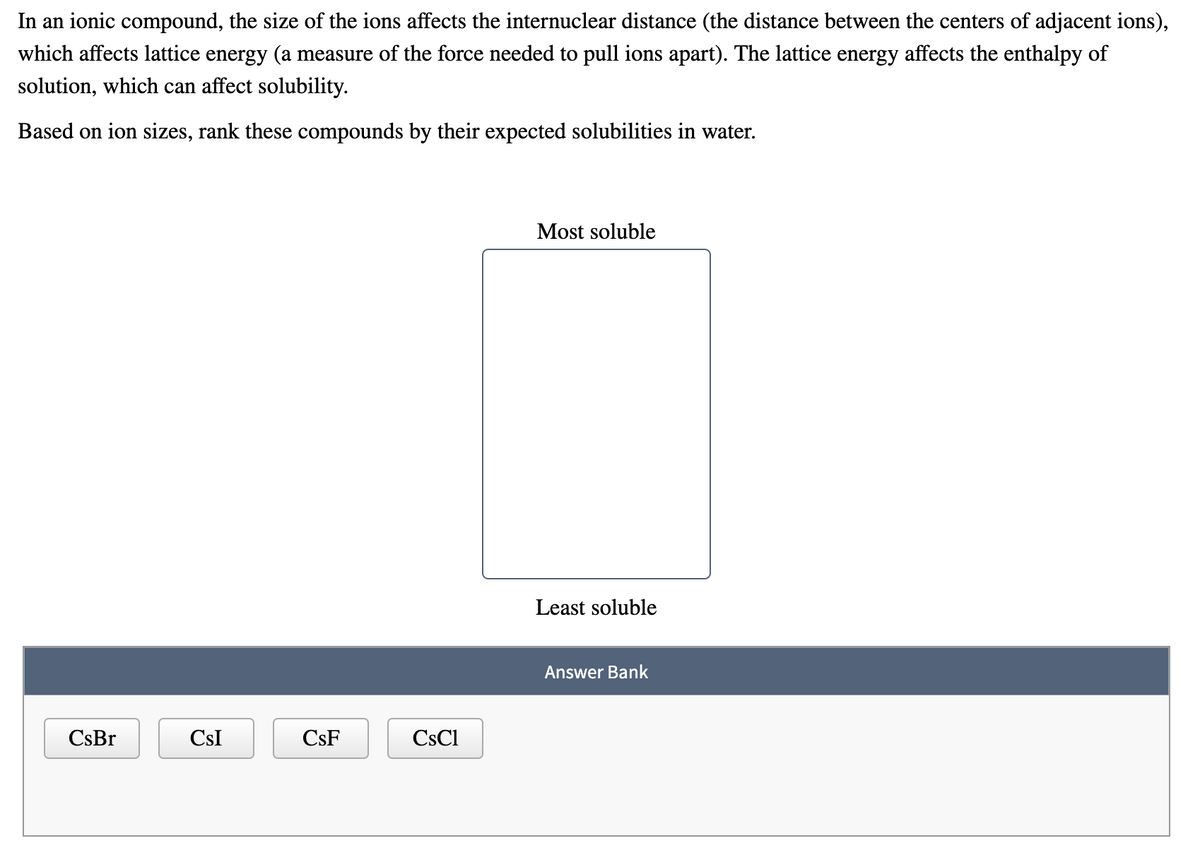
Transcribed Image Text:In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions),
which affects lattice energy (a measure of the force needed to pull ions apart). The lattice energy affects the enthalpy of
solution, which can affect solubility.
Based on ion sizes, rank these compounds by their expected solubilities in water.
Most soluble
Least soluble
Answer Bank
CsBr
CsI
CsF
CsCl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning