In class, students were given the following problem: "Aluminum satellite dishes resist corrosion because aluminum reacts with oxygen gas (0₂) to form a coating of aluminum oxide (Al2O3) according to the following chemical equation: 4Al(s)+302(g) → 2Al2O3(s) What is the mass of 9.45 moles of Al 20 3? After correctly solving for the molar mass of Al2O3, Jacob set up the following conversion: 9.45 moles Al2O3* 102.2 grams of Al, 0, 1 mole Al, 0₁ Is this answer correct? = 0.0926 grams of Al2O3
In class, students were given the following problem: "Aluminum satellite dishes resist corrosion because aluminum reacts with oxygen gas (0₂) to form a coating of aluminum oxide (Al2O3) according to the following chemical equation: 4Al(s)+302(g) → 2Al2O3(s) What is the mass of 9.45 moles of Al 20 3? After correctly solving for the molar mass of Al2O3, Jacob set up the following conversion: 9.45 moles Al2O3* 102.2 grams of Al, 0, 1 mole Al, 0₁ Is this answer correct? = 0.0926 grams of Al2O3
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.110QP: Hydrogen cyanide, HCN, can be made by a two-step process. First, ammonia is reacted with O2 to give...
Related questions
Question
100%
Include a CLAIM: answer the question. For example, this answer is not correct.
Include EVIDENCE: Describe what you see in the calculation work that supports your claim.
In the answer the wrong conversion factor was used. When calculate mass we.....
Include REASONING: Explain WHY you selected that evidence to support your claim.
You should have a paragraph.

Transcribed Image Text:In class, students were given the following problem:
"Aluminum satellite dishes resist corrosion because aluminum reacts with oxygen gas (0₂) to form a
coating of aluminum oxide (Al2O3) according to the following chemical equation:
4Al(s) + 302(g) →2A/203(s)
What is the mass of 9.45 moles of Al 203?
After correctly solving for the molar mass of Al2O3, Jacob set up the following conversion:
1 mole AL, Q
9.45 moles Al₂O3× 102.2 grams of Al, 0,
Is this answer correct?
= 0.0926 grams of Al2O3
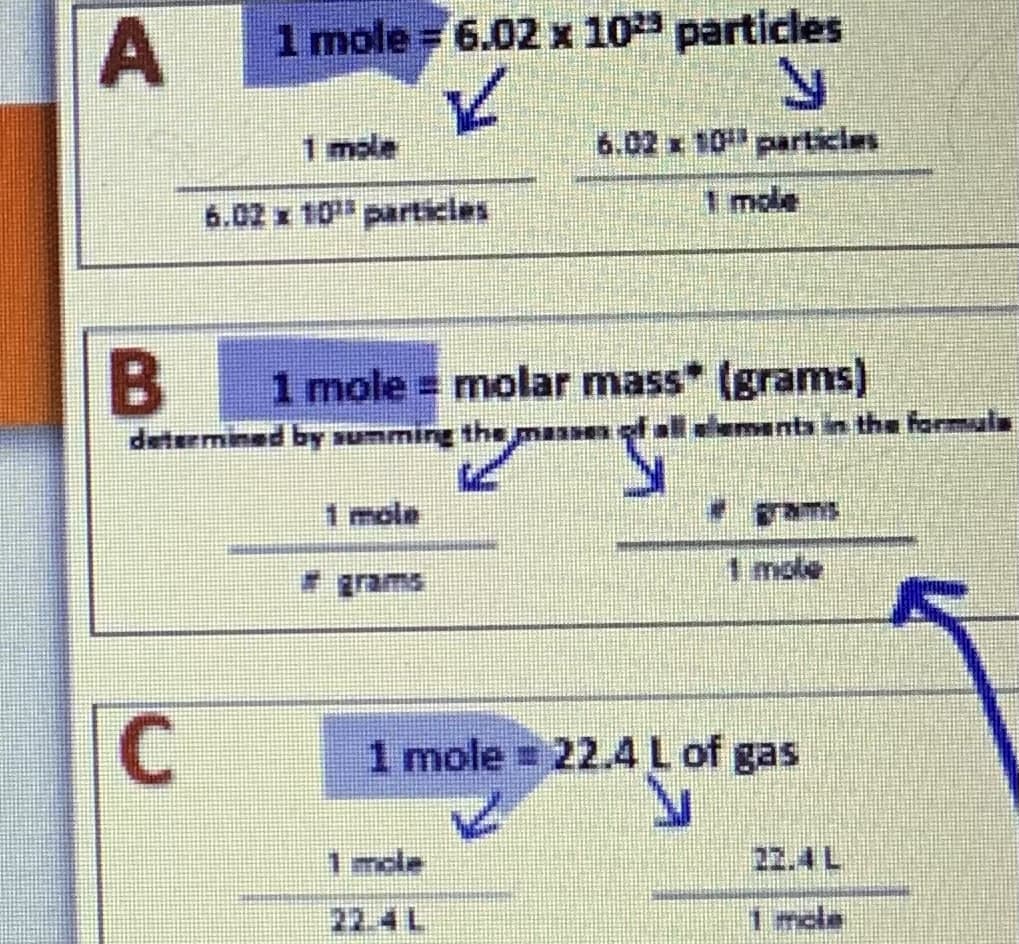
Transcribed Image Text:A
1 mole = 6.02 x 10²³ particles
✓
N
6.02 x 10 particles
1 mole
C
6.02 x 10¹ particles
B 1 mole = molar mass* (grams)
"S
determined by summing the ma 1 of all elements in the formula
1 mole = 22.4 L of gas
Inde
7241
22.4 L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning