In the chapter, we stated that the imine in this reaction is reduced. Verify that this is the case by calculating the oxidation state of the C and N atoms of the C=N group before and after the reaction has taken place. Does the imine gain electrons? If so, how many? NABH4 CH;OH
In the chapter, we stated that the imine in this reaction is reduced. Verify that this is the case by calculating the oxidation state of the C and N atoms of the C=N group before and after the reaction has taken place. Does the imine gain electrons? If so, how many? NABH4 CH;OH
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter23: Addition To A Carbonyl
Section: Chapter Questions
Problem 24E
Related questions
Question
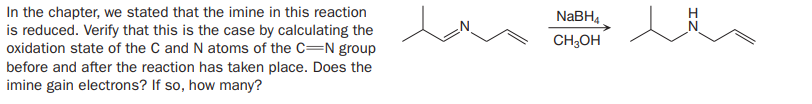
Transcribed Image Text:In the chapter, we stated that the imine in this reaction
is reduced. Verify that this is the case by calculating the
oxidation state of the C and N atoms of the C=N group
before and after the reaction has taken place. Does the
imine gain electrons? If so, how many?
NABH4
CH;OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
