In the figure, container A holds an ideal gas at a pressure of 4.26 x 105 Pa and a temperature of 300 K. It is connected by a thin tube (and a closed valve) to container B, with 4 times the volume of A. Container Bholds the same ideal gas at a pressure of 1.37 x 10° Pa and a temperature of 400 K. The valve is opened to allow the pressures to equalize, but the temperature of each container is maintained. What then is the pressure in the two containers? A B Number: i Unit:
In the figure, container A holds an ideal gas at a pressure of 4.26 x 105 Pa and a temperature of 300 K. It is connected by a thin tube (and a closed valve) to container B, with 4 times the volume of A. Container Bholds the same ideal gas at a pressure of 1.37 x 10° Pa and a temperature of 400 K. The valve is opened to allow the pressures to equalize, but the temperature of each container is maintained. What then is the pressure in the two containers? A B Number: i Unit:
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter10: Thermal Physics
Section: Chapter Questions
Problem 31P: (a) An ideal gas occupies a volume of 1.0 cm3 at 20.C and atmospheric pressure. Determine the number...
Related questions
Question
Please help me
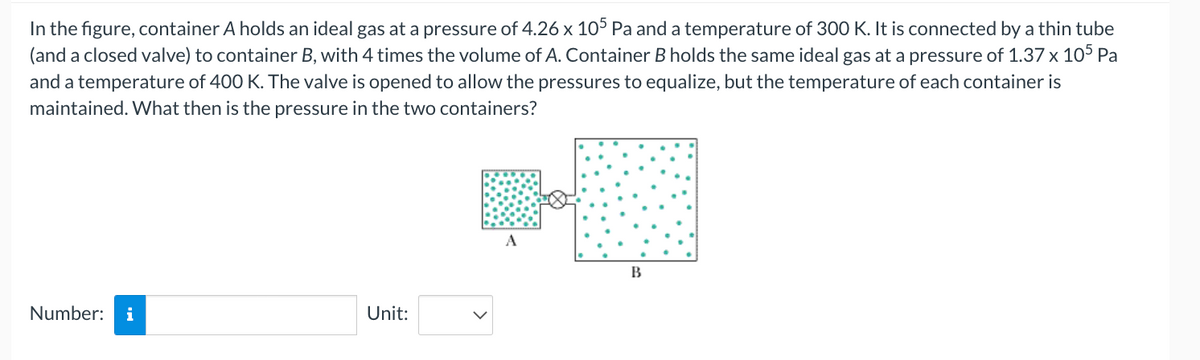
Transcribed Image Text:In the figure, container A holds an ideal gas at a pressure of 4.26 x 105 Pa and a temperature of 300 K. It is connected by a thin tube
(and a closed valve) to container B, with 4 times the volume of A. Container B holds the same ideal gas at a pressure of 1.37 x 105 Pa
and a temperature of 400 K. The valve is opened to allow the pressures to equalize, but the temperature of each container is
maintained. What then is the pressure in the two containers?
A
B
Number: i
Unit:
Expert Solution
Step 1
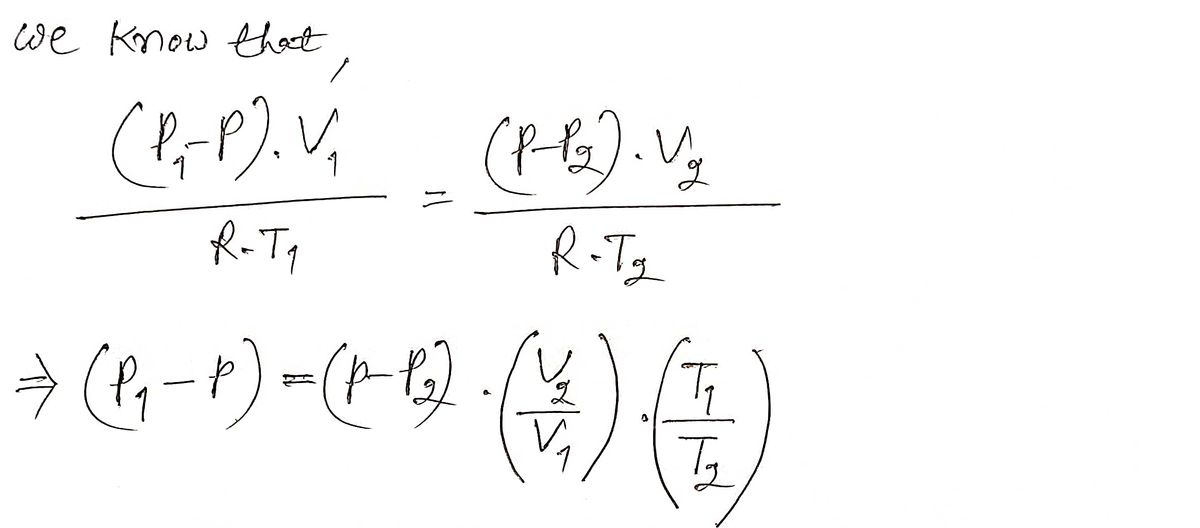
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning
