In the gold nanoparticle experiment, why did the two gold nanoparticle solutions have different maximums in their absorbance graphs? The first solution had a maxima at 579nm, and the second solution had a maxima at 531nm. Choose the BEST answer. Au Nanoparticle Solution 1 Au Nanoparcie Sounon S nh A he de w Sdn ean ghal ans erera ong O The concentration of tea affected the number of the nanoparticles, which affected the wavelength of light absorbed. O The concentration of tea affected the composition of the nanoparticles, which affected the wavelength of light absorbed. O The concentration of tea affected the size of the nanoparticles, which affected the wavelength of light absorbed. O There should have been no difference, this is experimental error.
In the gold nanoparticle experiment, why did the two gold nanoparticle solutions have different maximums in their absorbance graphs? The first solution had a maxima at 579nm, and the second solution had a maxima at 531nm. Choose the BEST answer. Au Nanoparticle Solution 1 Au Nanoparcie Sounon S nh A he de w Sdn ean ghal ans erera ong O The concentration of tea affected the number of the nanoparticles, which affected the wavelength of light absorbed. O The concentration of tea affected the composition of the nanoparticles, which affected the wavelength of light absorbed. O The concentration of tea affected the size of the nanoparticles, which affected the wavelength of light absorbed. O There should have been no difference, this is experimental error.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 6P
Related questions
Question
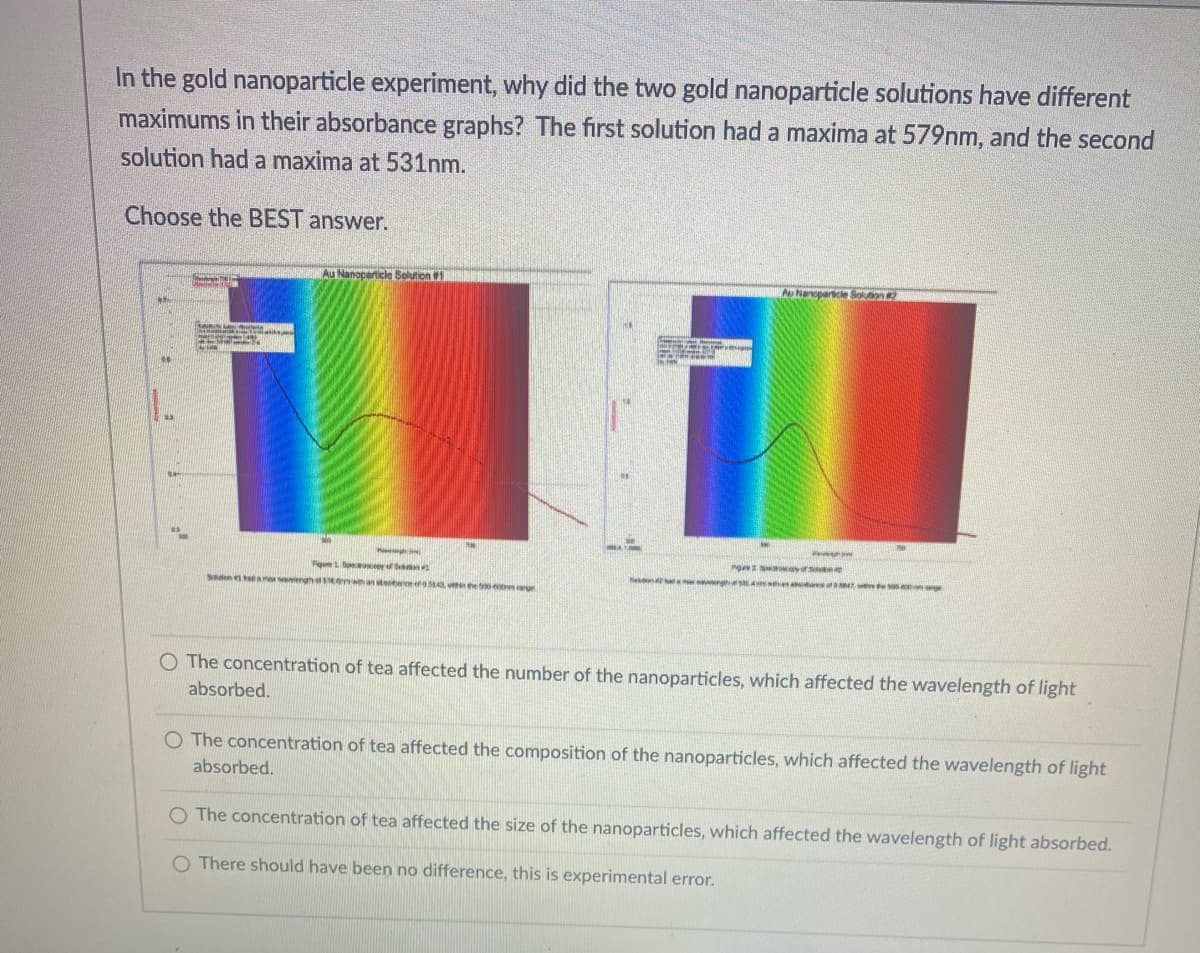
Transcribed Image Text:In the gold nanoparticle experiment, why did the two gold nanoparticle solutions have different
maximums in their absorbance graphs? The first solution had a maxima at 579nm, and the second
solution had a maxima at 531nm.
Choose the BEST answer.
Au Nanoparticle Solution1
Au Nancparncie Solunon 2
ng yr e
P1 be y S
Ston teeam ghalE whansttereera u e ong
Ne aa ngh A s w
O The concentration of tea affected the number of the nanoparticles, which affected the wavelength of light
absorbed.
O The concentration of tea affected the composition of the nanoparticles, which affected the wavelength of light
absorbed.
O The concentration of tea affected the size of the nanoparticles, which affected the wavelength of light absorbed.
O There should have been no difference, this is experimental error.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning