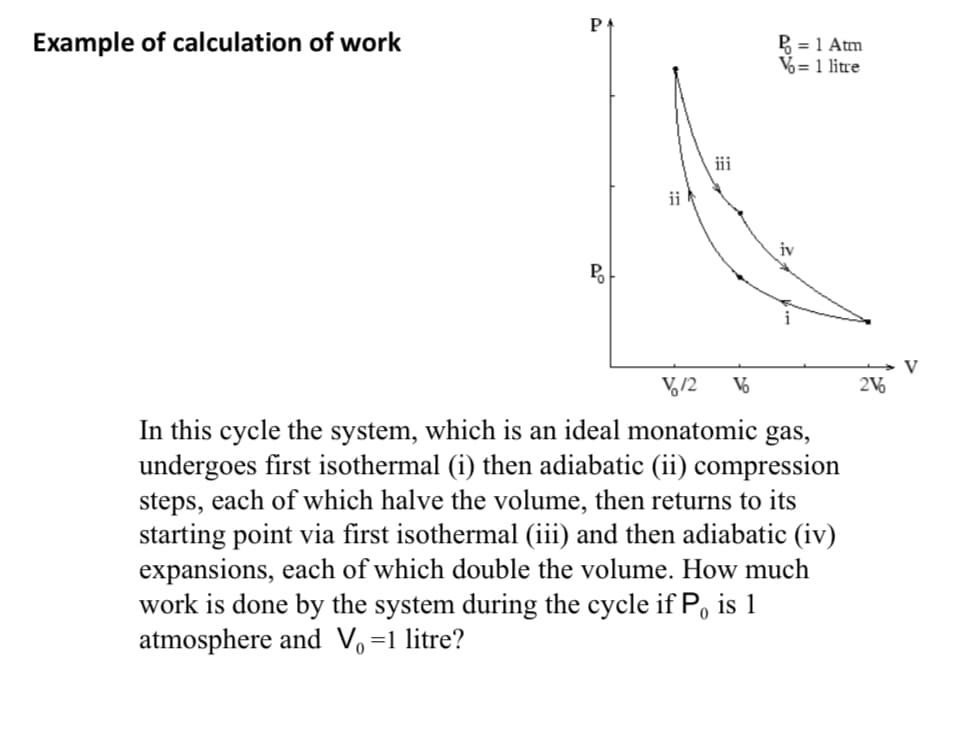
In this cycle the system, which is an ideal monatomic gas, undergoes first isothermal (i) then adiabatic (ii) compression steps, each of which halve the volume, then returns to its starting point via first isothermal (iii) and then adiabatic (iv) expansions, each of which double the volume. How much work is done by the system during the cycle if P, is 1 atmosphere and V,=1 litre?
In this cycle the system, which is an ideal monatomic gas, undergoes first isothermal (i) then adiabatic (ii) compression steps, each of which halve the volume, then returns to its starting point via first isothermal (iii) and then adiabatic (iv) expansions, each of which double the volume. How much work is done by the system during the cycle if P, is 1 atmosphere and V,=1 litre?
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 90AP: A cylinder containing three moles of a monatomic ideal gas is heated at a constant pressure of 2...
Related questions
Question
Transcribed Image Text:P
Example of calculation of work
P = 1 Atm
V%= 1 litre
iii
ii
P
V
V,12
V
In this cycle the system, which is an ideal monatomic gas,
undergoes first isothermal (i) then adiabatic (ii) compression
steps, each of which halve the volume, then returns to its
starting point via first isothermal (iii) and then adiabatic (iv)
expansions, each of which double the volume. How much
work is done by the system during the cycle if P, is 1
atmosphere and V, =1 litre?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning