Indicate the overall dipole for the molecule by selecting the arrow that points in the direction of the negative side of the dipole, or choose nonpolar if no dipole exists.
Indicate the overall dipole for the molecule by selecting the arrow that points in the direction of the negative side of the dipole, or choose nonpolar if no dipole exists.
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 22STP
Related questions
Concept explainers
Bond Parameters
Many factors decide the covalent bonding between atoms. Some of the bond parameters are bond angle, bond order, enthalpy, bond length, etc. These parameters decide what kind of bond will form in atoms. Hence it is crucial to understand these parameters in detail and understand how changing these parameters affects the kind of bonding or various characteristics.
Bond Dissociation Energy
The tendency of an atom to attract an electron is known as its electronegativity.
Question
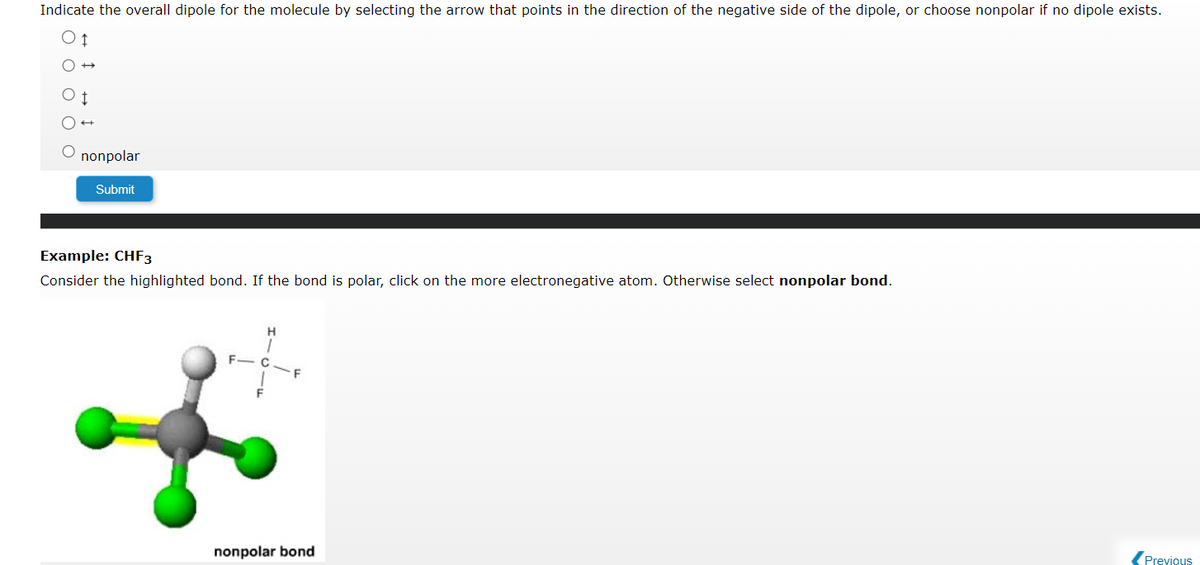
Transcribed Image Text:Indicate the overall dipole for the molecule by selecting the arrow that points in the direction of the negative side of the dipole, or choose nonpolar if no dipole exists.
Of
$
nonpolar
Submit
Example: CHF3
Consider the highlighted bond. If the bond is polar, click on the more electronegative atom. Otherwise select nonpolar bond.
F- C
ag
F
nonpolar bond
Previous
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning