(Involving a Linear Equation in x) The reaction CO(g) - H,O(g)= Co;(g) - H(g) has an equilibrium constant K, of 0.58 at 1000 °C. If a s0.0-L mixture of CO and HO has a concentration of each of 4.00x10 mol L, what amount (in moles) of each species will be present when the mixture reaches equilibrium mol CO
(Involving a Linear Equation in x) The reaction CO(g) - H,O(g)= Co;(g) - H(g) has an equilibrium constant K, of 0.58 at 1000 °C. If a s0.0-L mixture of CO and HO has a concentration of each of 4.00x10 mol L, what amount (in moles) of each species will be present when the mixture reaches equilibrium mol CO
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter27: Relative Stabilities Of Complex Ions And Precipitates Prepared From Solutions Of Copper(ii)
Section: Chapter Questions
Problem 2ASA
Related questions
Question
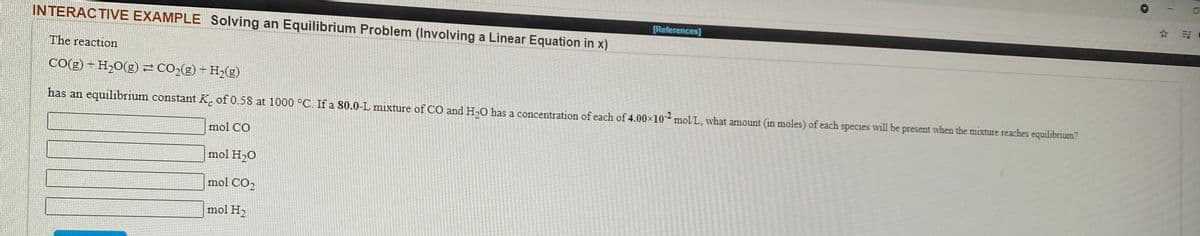
Transcribed Image Text:References)
INTERACTIVE EXAMPLE Solving an Equilibrium Problem (Involving a Linear Equation in x)
The reaction
CO(g) - H20(g)= co,(g) + H2(g)
has an equilibrium constant K. of 0.58 at 1000 °C. If a S0.0-L mixture of CO and H,O has a concentration of each of 4.00x10 mol L, what amount (in moles) of each species will be present when the mixture reaches equilibrium
mol CO
mol H20
mol CO2
mol H,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,