Item Mass of empty weigh boat Mass of mixture and weigh boat Total Mass of sample of Iron, Salt, and Sand Mass of sample AFTER iron is removed (simply salt and sand) Experimental Mass of Iron Obtained (show how 25.00-18.27= you are calculating this as well) Mass of filter paper Mass of filter paper and sand after drying Experimental Mass of sand (show how you are calculating this as wel!! Mass of empty beaker and aluminum foil lid Mass of beaker, foil, and crystalized salt after dry and cooled. Experimental Mass of Salt (show how you are calculating this as well) Your Percent by Mass of Iron in YOUR Mixture Your Percent by Mass of Sand in YOUR Mixture Your Percent by Mass of Salt in YOUR Mixture Percent Error for % Iron based on Theoretical Percent Error for % Sand based on Theoretical Percent Error for % Salt based on Theoretical 2.579 25.80g 23.23 18.279 7.53g 10.37-55= Recording ng 55g 10.37 9.829 79.919 85.619 5.7g 8561-29-91: 85.61-79.91= 32.41% 42.27% 24.54% Theoretical: 24.0% Fe Theoretical: 54.0% Sand Theoretical: 22.0% Salt
Item Mass of empty weigh boat Mass of mixture and weigh boat Total Mass of sample of Iron, Salt, and Sand Mass of sample AFTER iron is removed (simply salt and sand) Experimental Mass of Iron Obtained (show how 25.00-18.27= you are calculating this as well) Mass of filter paper Mass of filter paper and sand after drying Experimental Mass of sand (show how you are calculating this as wel!! Mass of empty beaker and aluminum foil lid Mass of beaker, foil, and crystalized salt after dry and cooled. Experimental Mass of Salt (show how you are calculating this as well) Your Percent by Mass of Iron in YOUR Mixture Your Percent by Mass of Sand in YOUR Mixture Your Percent by Mass of Salt in YOUR Mixture Percent Error for % Iron based on Theoretical Percent Error for % Sand based on Theoretical Percent Error for % Salt based on Theoretical 2.579 25.80g 23.23 18.279 7.53g 10.37-55= Recording ng 55g 10.37 9.829 79.919 85.619 5.7g 8561-29-91: 85.61-79.91= 32.41% 42.27% 24.54% Theoretical: 24.0% Fe Theoretical: 54.0% Sand Theoretical: 22.0% Salt
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 56QAP: Magnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its...
Related questions
Question
Please help
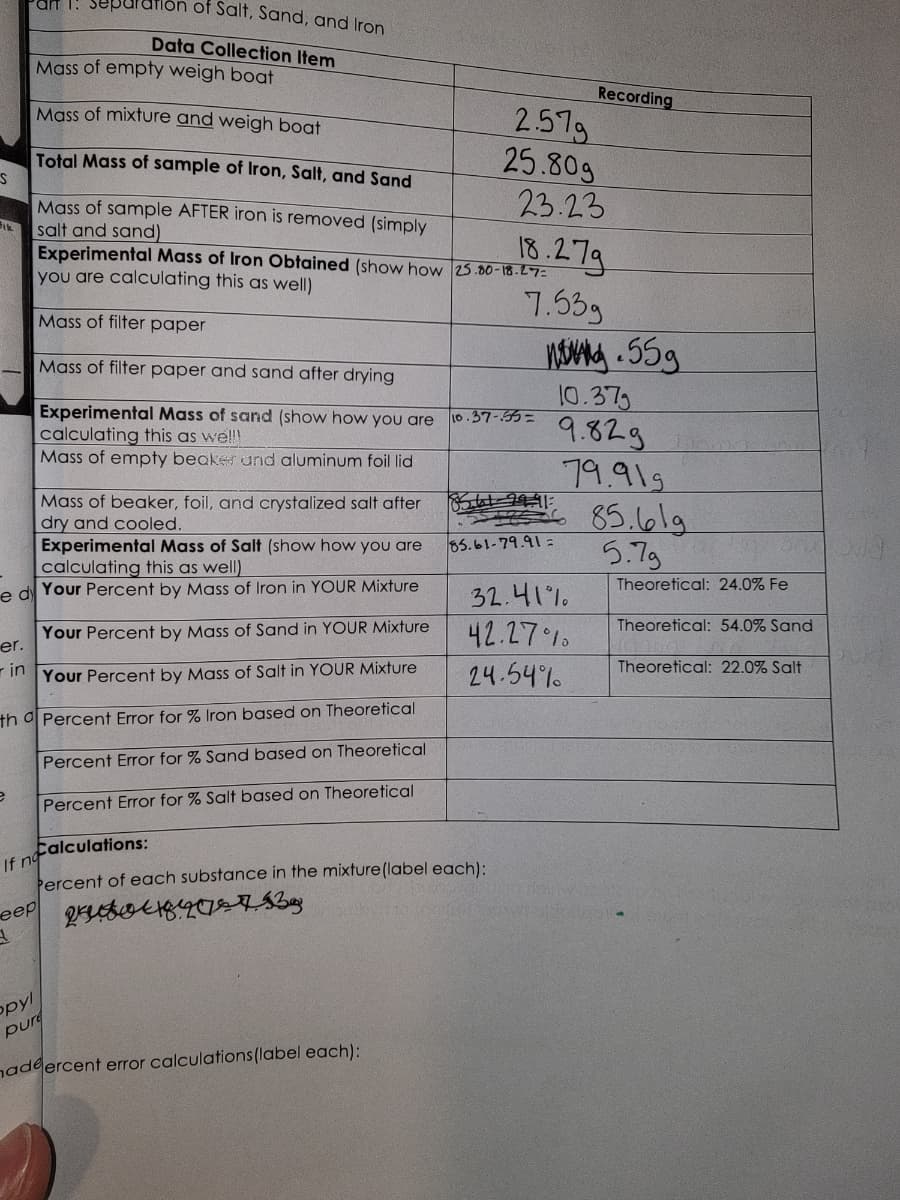
Transcribed Image Text:S
Fik
e
ar 1. separ
eep
3.
on of Salt, Sand, and Iron
Data Collection Item
Mass of empty weigh boat
Mass of mixture and weigh boat
Total Mass of sample of Iron, Salt, and Sand
Mass of sample AFTER iron is removed (simply
salt and sand)
Mass of beaker, foil, and crystalized salt after
dry and cooled.
Experimental Mass of Salt (show how you are
calculating this as well)
e dy Your Percent by Mass of Iron in YOUR Mixture
er.
Your Percent by Mass of Sand in YOUR Mixture
in Your Percent by Mass of Salt in YOUR Mixture
th a Percent Error for % Iron based on Theoretical
Percent Error for % Sand based on Theoretical
Percent Error for % Salt based on Theoretical
Calculations:
If no
Percent of each substance in the mixture (label each):
24827. 13.
Experimental Mass of Iron Obtained (show how 25.00-18.27=
you are calculating this as well)
Mass of filter paper
Mass of filter paper and sand after drying
Experimental Mass of sand (show how you are
calculating this as wel!!
Mass of empty beaker und aluminum foil lid
opyl
pure
hadeercent error calculations (label each):
2.579
25.80g
10.37-55=
23.23
18.279
7.53g
leotat
Recording
8561-99-91
85.61-79.91:
n.55g
10.37g
9.829
79.919
32.41%
42.27%
24.54%
واما.85
5.7g
Theoretical: 24.0% Fe
Theoretical: 54.0% Sand
Theoretical: 22.0% Salt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning