José performs an experiment to determine how much sugar can be dissolved in 250 milliliters (ml) of water at 25°C. He adds 50 grams (g) of sugar at a time to the water while stirming and observes the solution After adding sugar the eleventh tene (550 g), he notes that undissolved sugar has settled to the bottom of the container. He stops adding sugar and ends his experiment. In his lab write-up he concludes that the solubility of sugar in water at 25°C is 500 g per 250 ml is his conclusion defensible and why or why not? O No, because he did not add sugar enough times Yes, because when he added more than 500 g it did not all dissolve. O No, because he does not know if any of the final portion of sugar dissolved Yes, because when he added the tenth portion (500 g) all of it dissolved
José performs an experiment to determine how much sugar can be dissolved in 250 milliliters (ml) of water at 25°C. He adds 50 grams (g) of sugar at a time to the water while stirming and observes the solution After adding sugar the eleventh tene (550 g), he notes that undissolved sugar has settled to the bottom of the container. He stops adding sugar and ends his experiment. In his lab write-up he concludes that the solubility of sugar in water at 25°C is 500 g per 250 ml is his conclusion defensible and why or why not? O No, because he did not add sugar enough times Yes, because when he added more than 500 g it did not all dissolve. O No, because he does not know if any of the final portion of sugar dissolved Yes, because when he added the tenth portion (500 g) all of it dissolved
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 53QAP: Magnesium sulfate (MgSO4) has a solubility of 38.9 g/ 100 g H2O at 30C. A solution is prepared by...
Related questions
Question
100%
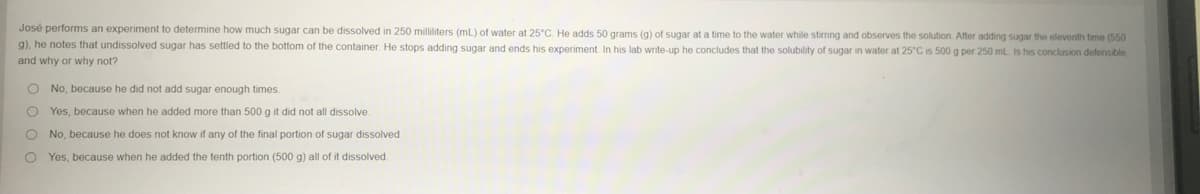
Transcribed Image Text:José performs an experiment to determine how much sugar can be dissolved in 250 milliliters (mL) of water at 25°C. He adds 50 grams (g) of sugar at a time to the water while stirring and observes the solution After adding sugar the eleventh tene (550
g), he notes that undissolved sugar has settled to the bottom of the container. He stops adding sugar and ends his experiment. In his lab write-up he concludes that the solubility of sugar in water at 25°C is 500 g per 250 ml. is his conclusion defensible,
and why or why not?
O No, because he did not add sugar enough times.
Yes, because when he added more than 500 g it did not all dissolve.
O No, because he does not know if any of the final portion of sugar dissolved.
O Yes, because when he added the tenth portion (500 g) all of it dissolved.
Expert Solution
Introduction
Solubility of a compound is the fraction of its concentration that dissolved in a solvent.
Solubility of compound depends on the nature of solvent and solute ,also on the temparature and pressure.
Solubility can be theoretically determined from the Ksp values and concentrations.
Experimentally solubilty can be determined simply by disoolving a constant amount of the solute at regular times to the solvent and observe it and also note down the settled amount.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning