kindly write a brief description of where each fragment peak originated from the parent ion. example: Formation of m/z 79 ion: [CH3CH2Br]+ ===> [79Br]^+ + CH2CH3. ( or a diagram of each bond breaking / forming could also work )
kindly write a brief description of where each fragment peak originated from the parent ion. example: Formation of m/z 79 ion: [CH3CH2Br]+ ===> [79Br]^+ + CH2CH3. ( or a diagram of each bond breaking / forming could also work )
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter17: Applications Of Infrared Spectrometry
Section: Chapter Questions
Problem 17.6QAP
Related questions
Question
kindly write a brief description of where each fragment peak originated from the parent ion. example: Formation of m/z 79 ion:
[CH3CH2Br]+ ===> [79Br]^+ + CH2CH3. ( or a diagram of each bond breaking / forming could also work )
![Parent ion is [CH3CH2B1]*•
Rel. Intensity
Proposed Fragment
[CH2]**
[CH3]*
[CHC]*.
[CHCH]*.
[CHCH2]*
14
3.9
15
3.6
25
26
9.6
43
27
94
29
[CH2CH2]*
[CH3CH2]*
[Br79]*.
[Br8]+.
28
29
100
Base peak
79
8.4
81
8.2
isotopic peak
[CH2B179]*.
[CH2Br81]*.
[CH3CH2B179]**
[CH3CH2B1®1]*.
93
95
4.3
3.6
isotopic peak
Parent ion peak (or) Molecular ion peak
M+2 isotopic peak
108
60.4
110
59.1](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd249dfc9-61b5-4487-bf6f-c6879951847e%2F7b8d2a3c-ae8f-4c73-84b6-382409e79626%2F72u6h59_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Parent ion is [CH3CH2B1]*•
Rel. Intensity
Proposed Fragment
[CH2]**
[CH3]*
[CHC]*.
[CHCH]*.
[CHCH2]*
14
3.9
15
3.6
25
26
9.6
43
27
94
29
[CH2CH2]*
[CH3CH2]*
[Br79]*.
[Br8]+.
28
29
100
Base peak
79
8.4
81
8.2
isotopic peak
[CH2B179]*.
[CH2Br81]*.
[CH3CH2B179]**
[CH3CH2B1®1]*.
93
95
4.3
3.6
isotopic peak
Parent ion peak (or) Molecular ion peak
M+2 isotopic peak
108
60.4
110
59.1
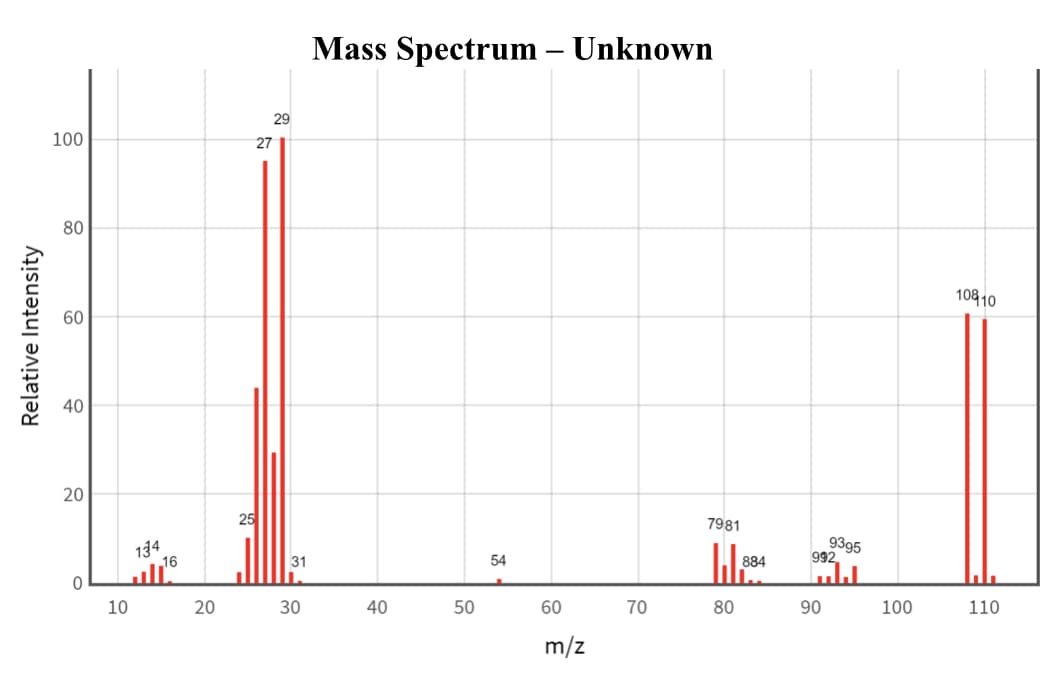
Transcribed Image Text:Mass Spectrum – Unknown
29
100
27
10& 10
60
40
20
25
7981
134
16
9395
992,
31
54
884
10
20
30
40
50
60
70
80
90
100
110
m/z
Relative Intensity
80
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
