Known Molarity of NaOH (from the bottle), M 0DLO897 mo1lL Trial 1 Trial 2 Trial 3 Volume of vinegar in flask, mL 10.00 mL 10.00 mL 10.00 ml Volume of vinegar in flask, L (watch sig fig) Just change mL above to L 1005 ML 15029ML 1549ML 251ML2077ML. Final burette reading, mL Initial burette reading, mL Volume of NaOH used, mL (subtract initial from final) Molarity of HC2H3O2, M (calculate this below for all 3 trials, then write answers in the boxes) Average Molarity of HC2H3O2, M Show your Molarity calculations for all three trials below. Watch the Post lab video for instructions. Dut the final answer for molarity of the vinegar in the table above. It will be a small number.
Known Molarity of NaOH (from the bottle), M 0DLO897 mo1lL Trial 1 Trial 2 Trial 3 Volume of vinegar in flask, mL 10.00 mL 10.00 mL 10.00 ml Volume of vinegar in flask, L (watch sig fig) Just change mL above to L 1005 ML 15029ML 1549ML 251ML2077ML. Final burette reading, mL Initial burette reading, mL Volume of NaOH used, mL (subtract initial from final) Molarity of HC2H3O2, M (calculate this below for all 3 trials, then write answers in the boxes) Average Molarity of HC2H3O2, M Show your Molarity calculations for all three trials below. Watch the Post lab video for instructions. Dut the final answer for molarity of the vinegar in the table above. It will be a small number.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 114AP
Related questions
Question
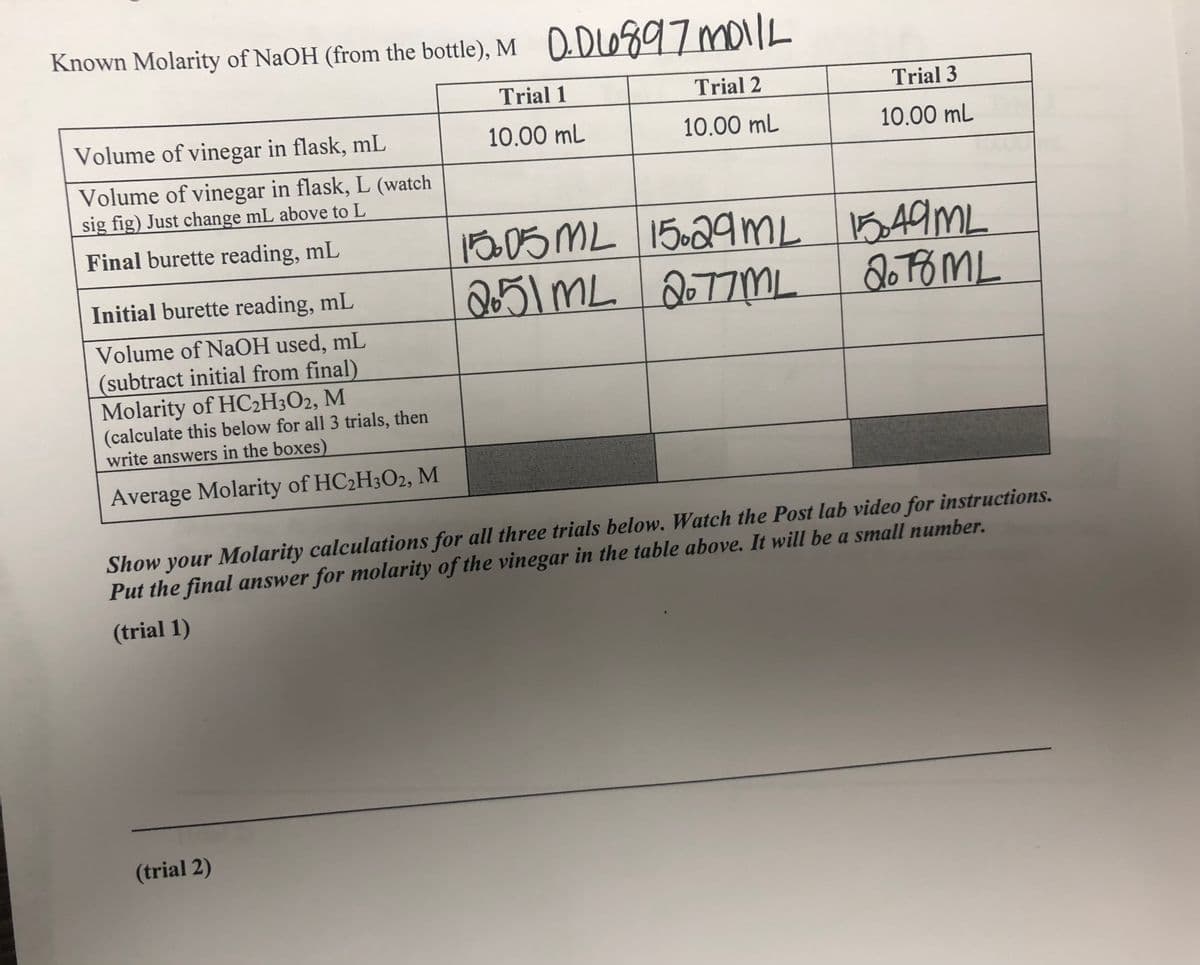
Transcribed Image Text:Known Molarity of NaOH (from the bottle), M 0.DLO897moilk
Trial 1
Trial 2
Trial 3
Volume of vinegar in flask, mL
10.00 mL
10.00 mL
10.00 ml
Volume of vinegar in flask, L (watch
sig fig) Just change mL above to L
13005 ML 15.29mL 549ML
15.29ML
Final burette reading, mL
251 mL 2077ML
Initial burette reading, mL
Volume of NaOH used, mL
(subtract initial from final)
Molarity of HC2H3O2, M
(calculate this below for all 3 trials, then
write answers in the boxes)
Average Molarity of HC2H3O2, M
Show your Molarity calculations for all three trials below. Watch the Post lab video for instructions.
Put the final answer for molarity of the vinegar in the table above. It will be a small number.
(trial 1)
(trial 2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning