lege-CHEM 1020 - Spring20 - NGWENDSON Activities and Due Dates > Ch 7 HW Assignment Score: 42.6% Resources Give Up? O Hint Check Answer Kス Question 22 of 23 > Combining 0.256 mol Fe, O, with excess carbon produced 18.7 g Fe. Fe, 0, + 3C – 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? mol theoretical yield: What is the percent yield? terms of use contact us help about us careers privacy policy 13 6. Aa 59 APR 16 DD F12 DII F11 F10 F9 F8 F7 20 F6 F5 F4 F3 F2 delete %3D & %23 %24 CO
lege-CHEM 1020 - Spring20 - NGWENDSON Activities and Due Dates > Ch 7 HW Assignment Score: 42.6% Resources Give Up? O Hint Check Answer Kス Question 22 of 23 > Combining 0.256 mol Fe, O, with excess carbon produced 18.7 g Fe. Fe, 0, + 3C – 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? mol theoretical yield: What is the percent yield? terms of use contact us help about us careers privacy policy 13 6. Aa 59 APR 16 DD F12 DII F11 F10 F9 F8 F7 20 F6 F5 F4 F3 F2 delete %3D & %23 %24 CO
Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.25QAP
Related questions
Question
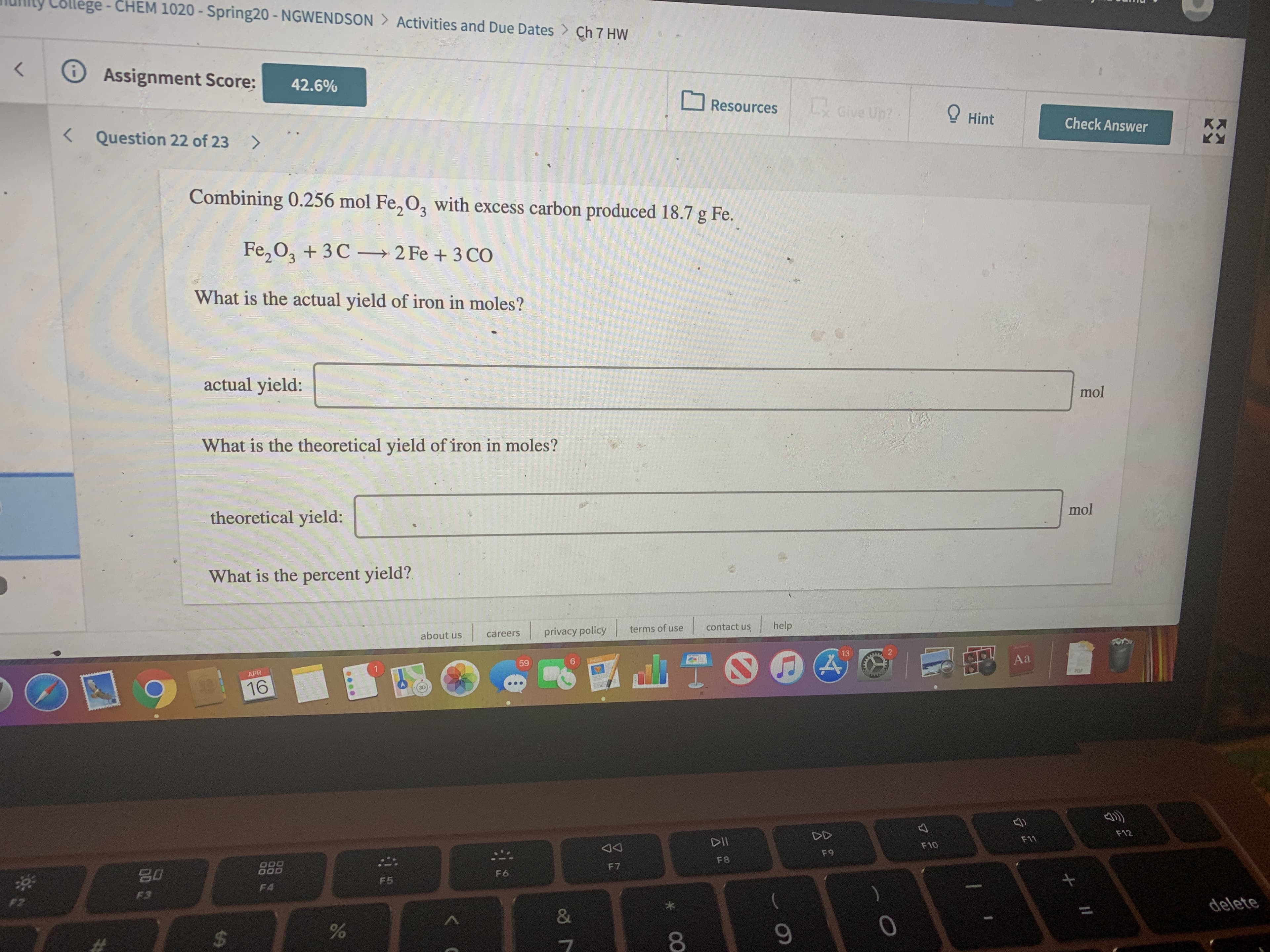
Transcribed Image Text:lege-CHEM 1020 - Spring20 - NGWENDSON Activities and Due Dates > Ch 7 HW
Assignment Score:
42.6%
Resources
Give Up?
O Hint
Check Answer
Kス
Question 22 of 23 >
Combining 0.256 mol Fe, O, with excess carbon produced 18.7 g Fe.
Fe, 0, + 3C – 2 Fe + 3 CO
What is the actual yield of iron in moles?
actual yield:
mol
What is the theoretical yield of iron in moles?
mol
theoretical yield:
What is the percent yield?
terms of use
contact us
help
about us
careers
privacy policy
13
6.
Aa
59
APR
16
DD
F12
DII
F11
F10
F9
F8
F7
20
F6
F5
F4
F3
F2
delete
%3D
&
%23
%24
CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
