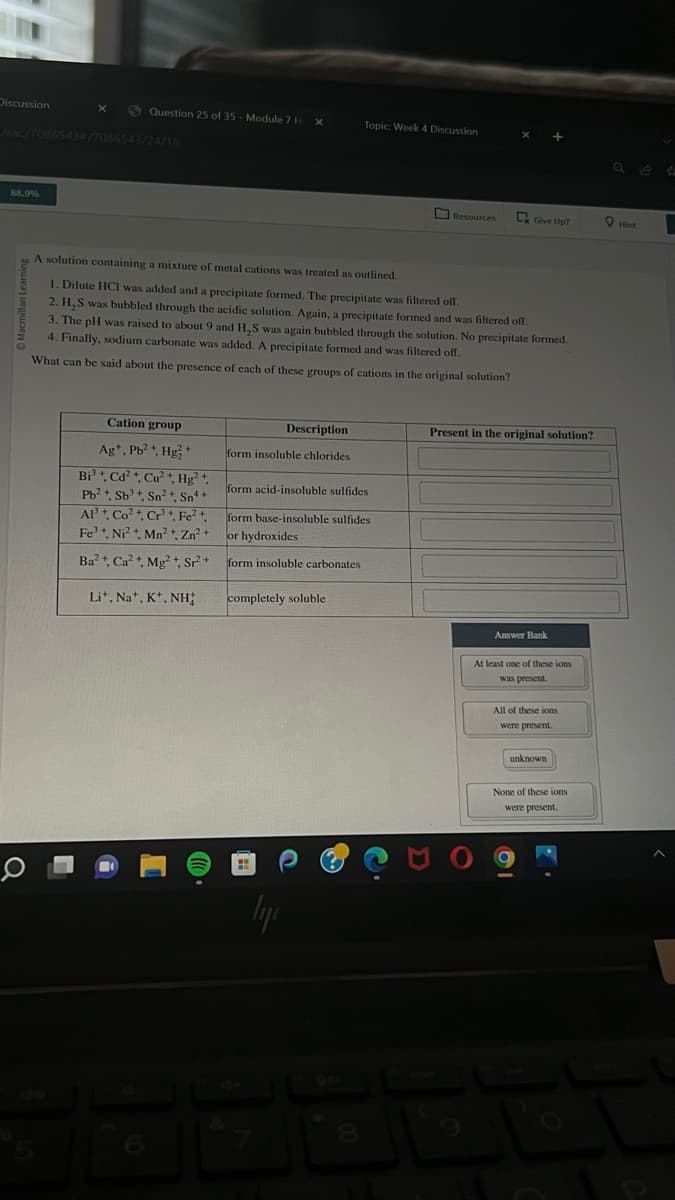
Macmillan Learning O A solution containing a mixture of metal cations was treated as outlined. 1. Dilute HCI was added and a precipitate formed. The precipitate was filtered off. 2. H₂S was bubbled through the acidic solution. Again, a precipitate formed and was filtered off. 3. The pH was raised to about 9 and H₂S was again bubbled through the solution. No precipitate formed. 4. Finally, sodium carbonate was added. A precipitate formed and was filtered off. What can be said about the presence of each of these groups of cations in the original solution? Cation group Ag+, Pb²+, Hg2+ Bi³+, Cd²+, Cu²+, Hg²+, Pb²+, Sb +, Sn²+, Sn+ AP+, Co²+, Cr, Fe²+, Fe³+, Ni²+, Mn²+, Zn²+ Ba²+, Ca²+, Mg²+, SP2²+ Li+, Na+, K+, NH; Description form insoluble chlorides form acid-insoluble sulfides form base-insoluble sulfides or hydroxides form insoluble carbonates completely soluble Present in the original solution? Answer Bank At least one of these ions was present. All of these ions were present. unknown None of these ions were present.
Macmillan Learning O A solution containing a mixture of metal cations was treated as outlined. 1. Dilute HCI was added and a precipitate formed. The precipitate was filtered off. 2. H₂S was bubbled through the acidic solution. Again, a precipitate formed and was filtered off. 3. The pH was raised to about 9 and H₂S was again bubbled through the solution. No precipitate formed. 4. Finally, sodium carbonate was added. A precipitate formed and was filtered off. What can be said about the presence of each of these groups of cations in the original solution? Cation group Ag+, Pb²+, Hg2+ Bi³+, Cd²+, Cu²+, Hg²+, Pb²+, Sb +, Sn²+, Sn+ AP+, Co²+, Cr, Fe²+, Fe³+, Ni²+, Mn²+, Zn²+ Ba²+, Ca²+, Mg²+, SP2²+ Li+, Na+, K+, NH; Description form insoluble chlorides form acid-insoluble sulfides form base-insoluble sulfides or hydroxides form insoluble carbonates completely soluble Present in the original solution? Answer Bank At least one of these ions was present. All of these ions were present. unknown None of these ions were present.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter19: Transitition Metals, Coordination Chemistry And Metallurgy
Section: Chapter Questions
Problem 19.5QE
Related questions
Question
Transcribed Image Text:Discussion
X
88.9%
Question 25 of 35-Module 7 H X
/sac/7086543#/7086543/24/16
Cation group
Ag+, Pb²+, Hg2+
Bi³+, Cd²+, Cu²+, Hg²+,
Pb²+, Sb³ +, Sn²+, Sn¹+
Al³+, Co²+, Cr³+. Fe²+
Fe³+, Ni²+, Mn²+, Zn²+
Ba²+, Ca²+, Mg2+, S²+
Lit, Na+, K+, NH
A solution containing a mixture of metal cations was treated as outlined.
1. Dilute HCI was added and a precipitate formed. The precipitate was filtered off.
2. H₂S was bubbled through the acidic solution. Again, a precipitate formed and was filtered off.
3. The pH was raised to about 9 and H,S was again bubbled through the solution. No precipitate formed.
4. Finally, sodium carbonate was added. A precipitate formed and was filtered off.
What can be said about the presence of each of these groups of cations in the original solution?
Description
form insoluble chlorides
form acid-insoluble sulfides
form base-insoluble sulfides
or hydroxides
form insoluble carbonates
completely soluble
Topic: Week 4 Discussion
lyi
32
Resources
Give Up?
Present in the original solution?
Answer Bank
At least one of these ions
was present.
All of these ions
were present.
unknown
None of these ions
were present.
Hint
☆
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning