Metal Sample Large 32.30 Triple-beam Balance Small Mass Beaker (g) Mass of Metal + Beaker (g) 110.74 Initial Volume (mL) Final Volume (mL) 32.30 67.22 63.8 55.5 70.5 58.7 Metal Sample Large 32.56 Electronic Balance Small Mass Beaker (g) Mass of Metal + Beaker (g) 89.06 Initial Volume (mL) Final Volume (mL) 32.56 53.71 59.9 55.2 65.0 57.1 Metal Sample Small 32.5760 32.5760 Mass of Metal + Beaker (g) 56.4270 56.5776 Analytical Balance Large Mass Beaker (g) Initial Volume (mL) Final Volume (mL) 56.2 56.2 58.2 57.8 The following data was obtained in the lab. Calculate the average density of the metal obtained on the electronic balance. 10.0 11 11.01 11.1
Metal Sample Large 32.30 Triple-beam Balance Small Mass Beaker (g) Mass of Metal + Beaker (g) 110.74 Initial Volume (mL) Final Volume (mL) 32.30 67.22 63.8 55.5 70.5 58.7 Metal Sample Large 32.56 Electronic Balance Small Mass Beaker (g) Mass of Metal + Beaker (g) 89.06 Initial Volume (mL) Final Volume (mL) 32.56 53.71 59.9 55.2 65.0 57.1 Metal Sample Small 32.5760 32.5760 Mass of Metal + Beaker (g) 56.4270 56.5776 Analytical Balance Large Mass Beaker (g) Initial Volume (mL) Final Volume (mL) 56.2 56.2 58.2 57.8 The following data was obtained in the lab. Calculate the average density of the metal obtained on the electronic balance. 10.0 11 11.01 11.1
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 2RCYU: A student checked the accuracy of two standard top-loading balances by testing them with a standard...
Related questions
Question
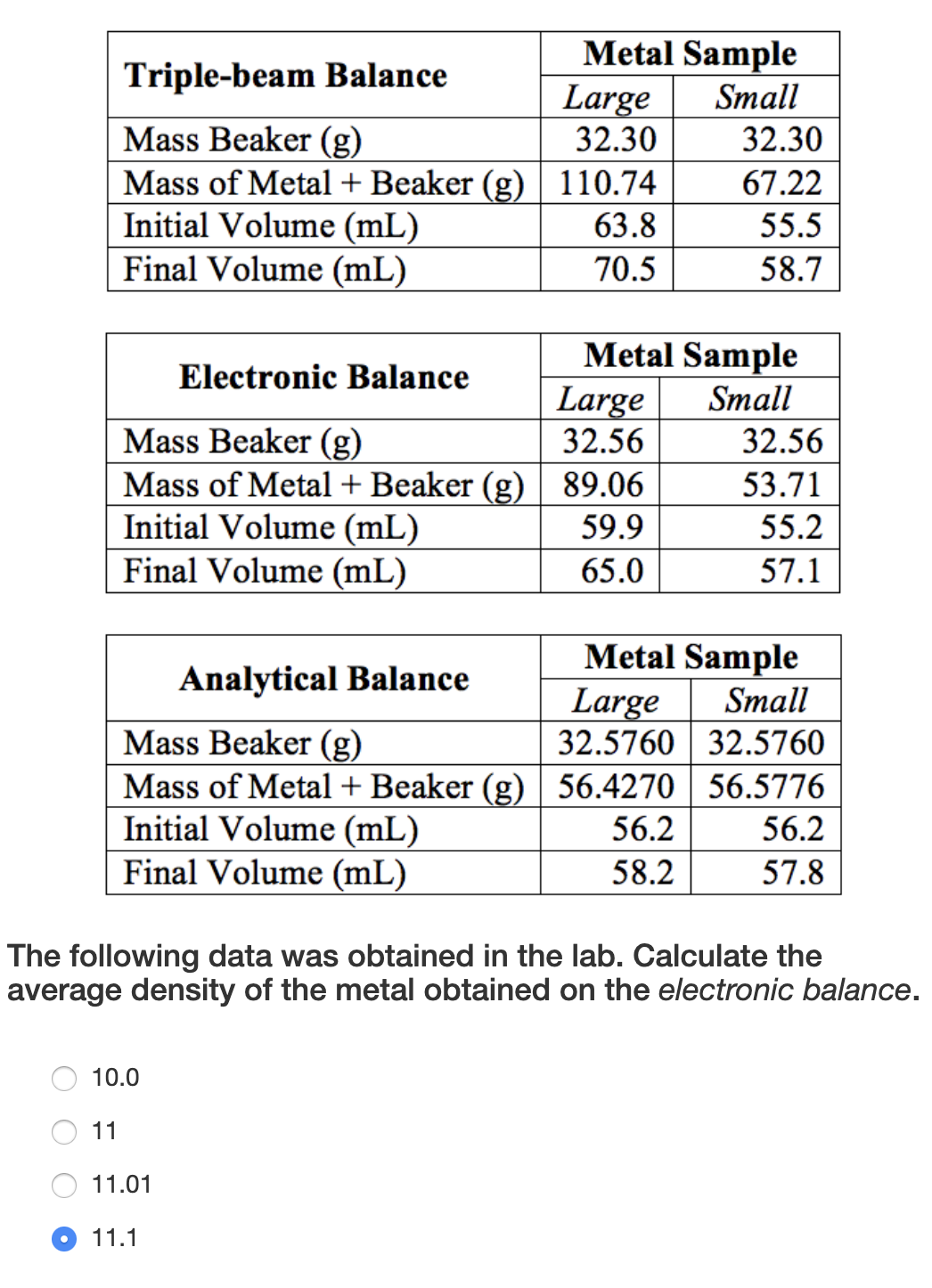
Transcribed Image Text:Metal Sample
Large
32.30
Triple-beam Balance
Small
Mass Beaker (g)
Mass of Metal + Beaker (g) 110.74
Initial Volume (mL)
Final Volume (mL)
32.30
67.22
63.8
55.5
70.5
58.7
Metal Sample
Large
32.56
Electronic Balance
Small
Mass Beaker (g)
Mass of Metal + Beaker (g) 89.06
Initial Volume (mL)
Final Volume (mL)
32.56
53.71
59.9
55.2
65.0
57.1
Metal Sample
Small
32.5760 32.5760
Mass of Metal + Beaker (g) 56.4270 56.5776
Analytical Balance
Large
Mass Beaker (g)
Initial Volume (mL)
Final Volume (mL)
56.2
56.2
58.2
57.8
The following data was obtained in the lab. Calculate the
average density of the metal obtained on the electronic balance.
10.0
11
11.01
11.1
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 10 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
