Metals are often plated with copper to increase their conductivity, or also due to the antibacterial properties of copper 3CU2 (aq) + 2Fes) —> 2Fезt (aq) + 3Cus) How long will it take to plate out 12 with a current of 9 amps in the above reaction? (Hint: write out the half-reactions and use the stoichiometric ratios; also, amp is 1 C/s, and of grams соpper C Faraday's constant is 96485 mol of e- How many amps are needed to precipitate out 19 copper in 6 seconds? of grams
Metals are often plated with copper to increase their conductivity, or also due to the antibacterial properties of copper 3CU2 (aq) + 2Fes) —> 2Fезt (aq) + 3Cus) How long will it take to plate out 12 with a current of 9 amps in the above reaction? (Hint: write out the half-reactions and use the stoichiometric ratios; also, amp is 1 C/s, and of grams соpper C Faraday's constant is 96485 mol of e- How many amps are needed to precipitate out 19 copper in 6 seconds? of grams
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
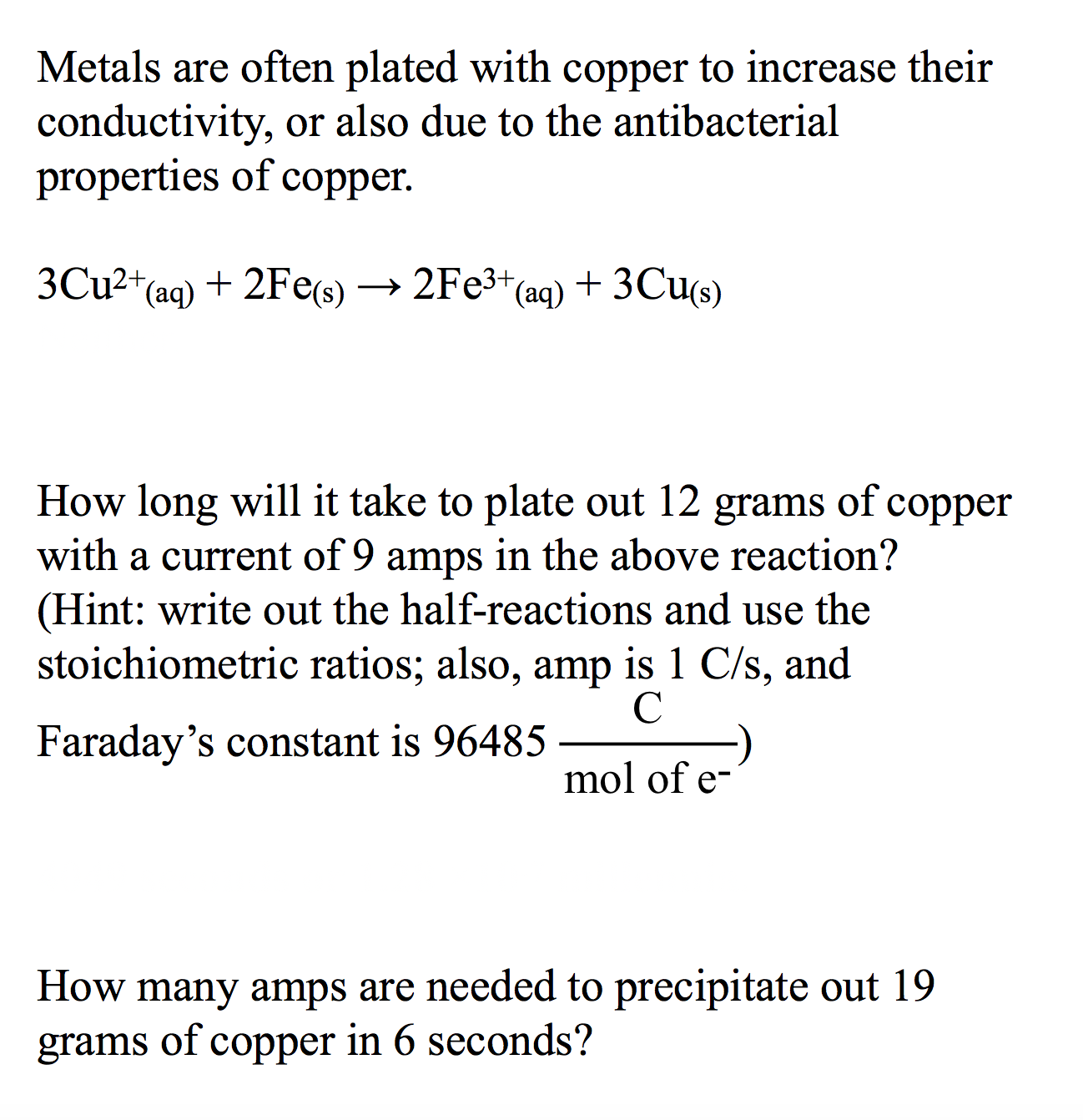
Transcribed Image Text:Metals are often plated with copper to increase their
conductivity, or also due to the antibacterial
properties of copper
3CU2 (aq)
+ 2Fes) —> 2Fезt (aq) + 3Cus)
How long will it take to plate out 12
with a current of 9 amps in the above reaction?
(Hint: write out the half-reactions and use the
stoichiometric ratios; also, amp is 1 C/s, and
of
grams
соpper
C
Faraday's constant is 96485
mol of e-
How many amps are needed to precipitate out 19
copper in 6 seconds?
of
grams
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

