Model 3 The Model: Dilution Process of dilution: add solvent to conc. soln. x X хх "concentrated" pure solvent "dilute" solution of solute X solution of solute X (X's are in motion.) (X's are in motion.) dilution formula: Mainal Vinitial "Minal Vánel (or Mi V - M; V) 1) When a solution is diluted, additional solvent is added to the more concentrated solution. How does the amount of solute in the initial (concentrated) solution compare to the amount of solute in the final (dilute) solution? 2) By referring to the model, justify the dilution formula. Explain why the product of the solution's initial concentration (molarity) and initial volume is equal to the product of the solution's final concentration and final volume.
Model 3 The Model: Dilution Process of dilution: add solvent to conc. soln. x X хх "concentrated" pure solvent "dilute" solution of solute X solution of solute X (X's are in motion.) (X's are in motion.) dilution formula: Mainal Vinitial "Minal Vánel (or Mi V - M; V) 1) When a solution is diluted, additional solvent is added to the more concentrated solution. How does the amount of solute in the initial (concentrated) solution compare to the amount of solute in the final (dilute) solution? 2) By referring to the model, justify the dilution formula. Explain why the product of the solution's initial concentration (molarity) and initial volume is equal to the product of the solution's final concentration and final volume.
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
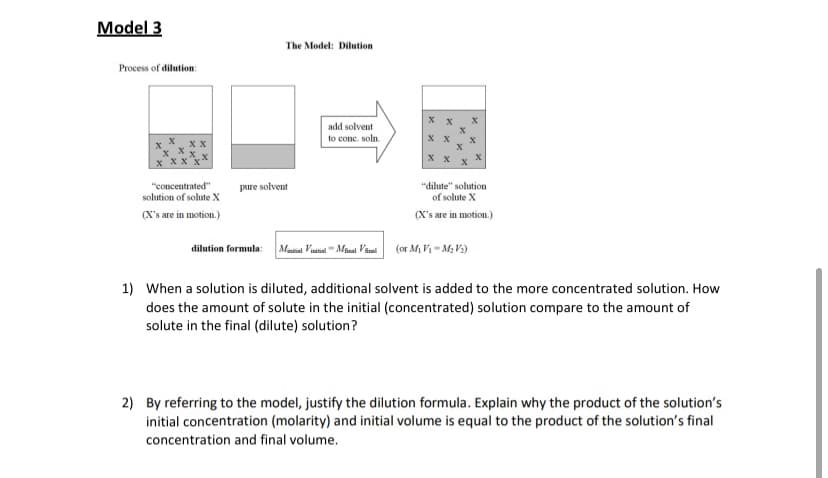
Transcribed Image Text:Model 3
The Model: Dilution
Process of dilution:
add solvent
to conc. soln.
хх
"concentrated"
solution of solute X
pure solvent
"dilute" solution
of solute X
(X's are in motion.)
(X's are in motion.)
dilution formula:
Maital Vinitial"Minal Viul
(or Mi V1 = M, V3)
1) When a solution is diluted, additional solvent is added to the more concentrated solution. How
does the amount of solute in the initial (concentrated) solution compare to the amount of
solute in the final (dilute) solution?
2) By referring to the model, justify the dilution formula. Explain why the product of the solution's
initial concentration (molarity) and initial volume is equal to the product of the solution's final
concentration and final volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT