Most of the coal available in the United States is bituminous or "soft" coal, which contains as much as 5% sulfur by weight. When this coal is burned, the sulfur reacts with oxygen to form sulfur dioxide gas. When the sulfur dioxide gas dissolves in raindrops, it forms sulfuric acid. This is how we get acid rain. Here is the reaction that produces sulfur dioxide gas when soft coal is burned: S,(s)+80,(g) 8so,(g) Suppose an engineer decides to study the rate of this reaction. She prepares four reaction vessels with 165.4 g of solid sulfur and 40.7 g of oxygen gas each. The volume and temperature of each vessel is shown in the table below. do Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. initial rate of vessel volume temperature reaction A 8.0 L 610. °C 4.0 L 610. °C 2.0 L 610. °C 1.0 L 610. °C
Most of the coal available in the United States is bituminous or "soft" coal, which contains as much as 5% sulfur by weight. When this coal is burned, the sulfur reacts with oxygen to form sulfur dioxide gas. When the sulfur dioxide gas dissolves in raindrops, it forms sulfuric acid. This is how we get acid rain. Here is the reaction that produces sulfur dioxide gas when soft coal is burned: S,(s)+80,(g) 8so,(g) Suppose an engineer decides to study the rate of this reaction. She prepares four reaction vessels with 165.4 g of solid sulfur and 40.7 g of oxygen gas each. The volume and temperature of each vessel is shown in the table below. do Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on. initial rate of vessel volume temperature reaction A 8.0 L 610. °C 4.0 L 610. °C 2.0 L 610. °C 1.0 L 610. °C
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 2.1ACP
Related questions
Question
Please give me the right answer
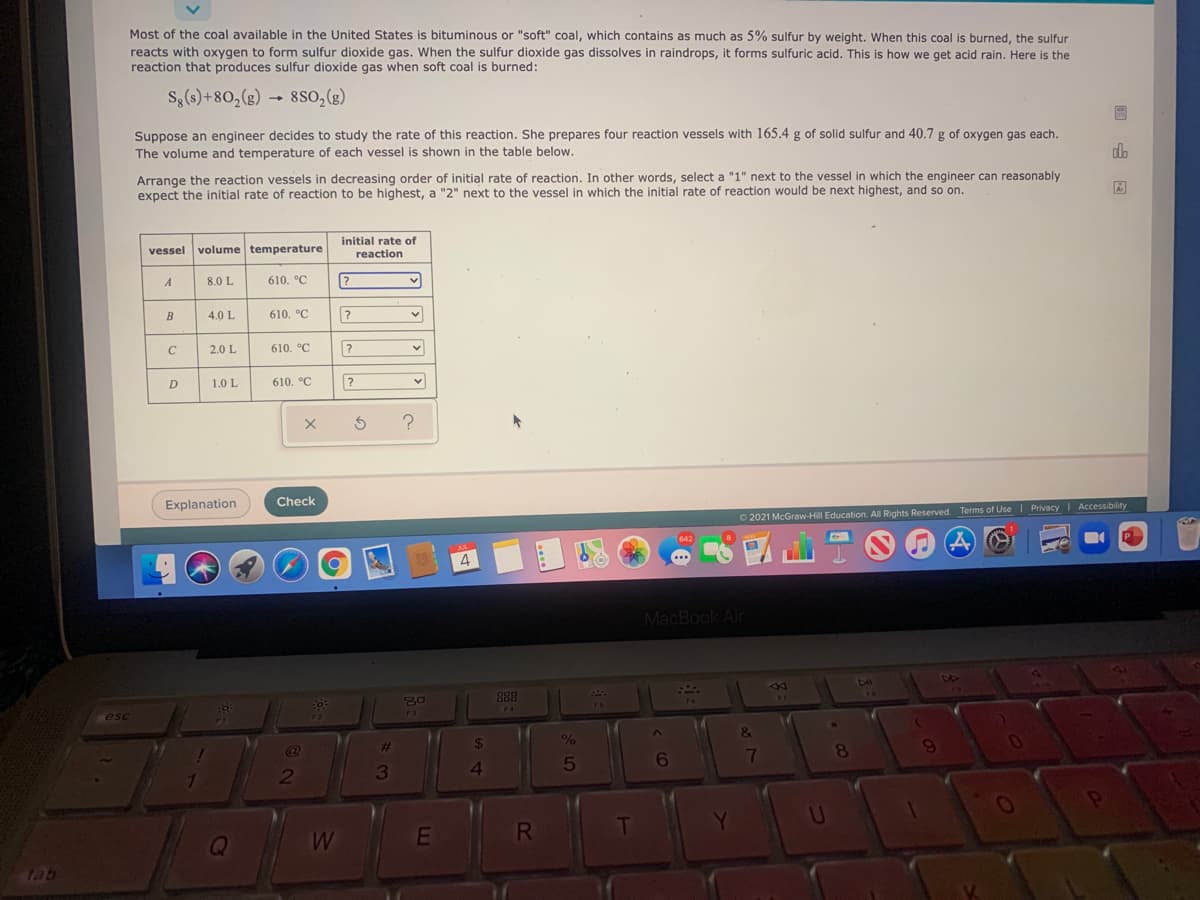
Transcribed Image Text:Most of the coal available in the United States is bituminous or "soft" coal, which contains as much as 5% sulfur by weight. When this coal is burned, the sulfur
reacts with oxygen to form sulfur dioxide gas. When the sulfur dioxide gas dissolves in raindrops, it forms sulfuric acid. This is how we get acid rain. Here is the
reaction that produces sulfur dioxide gas when soft coal is burned:
Sg(s)+80,(g)
8so,(e)
Suppose an engineer decides to study the rate of this reaction. She prepares four reaction vessels with 165.4 g of solid sulfur and 40.7 g of oxygen gas each.
The volume and temperature of each vessel is shown in the table below.
dlo
Arrange the reaction vessels in decreasing order of initial rate of reaction. In other words, select a "1" next to the vessel in which the engineer can reasonably
expect the initial rate of reaction to be highest, a "2" next to the vessel in which the initial rate of reaction would be next highest, and so on.
initial rate of
vessel
volume temperature
reaction
A
8.0 L
610. °C
B
4.0 L
610. °C
2.0 L
610. °C
D
1.0 L
610. °C
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessibility
4
MacBook Air
44
S0
888
FA
esc
%23
24
8.
6
7
2
3
RI
tab
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning