n=4 V In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. n=3 One transition between energy states of the hydrogen atom is represented by the picture on the left. n-2 Niels Bohr n=1 1. In this transition an electron moves from the n = level to the n = level. A. Absorbed 2. Energy is in this process. B. Emitted A. Closer to 3. The electron moves the nucleus. B. Further from
n=4 V In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. n=3 One transition between energy states of the hydrogen atom is represented by the picture on the left. n-2 Niels Bohr n=1 1. In this transition an electron moves from the n = level to the n = level. A. Absorbed 2. Energy is in this process. B. Emitted A. Closer to 3. The electron moves the nucleus. B. Further from
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.29PAE: 6.29 A mercury atom emits light at many wavelengths, two of which are at 435.8 and 546.1 nm. Both of...
Related questions
Question
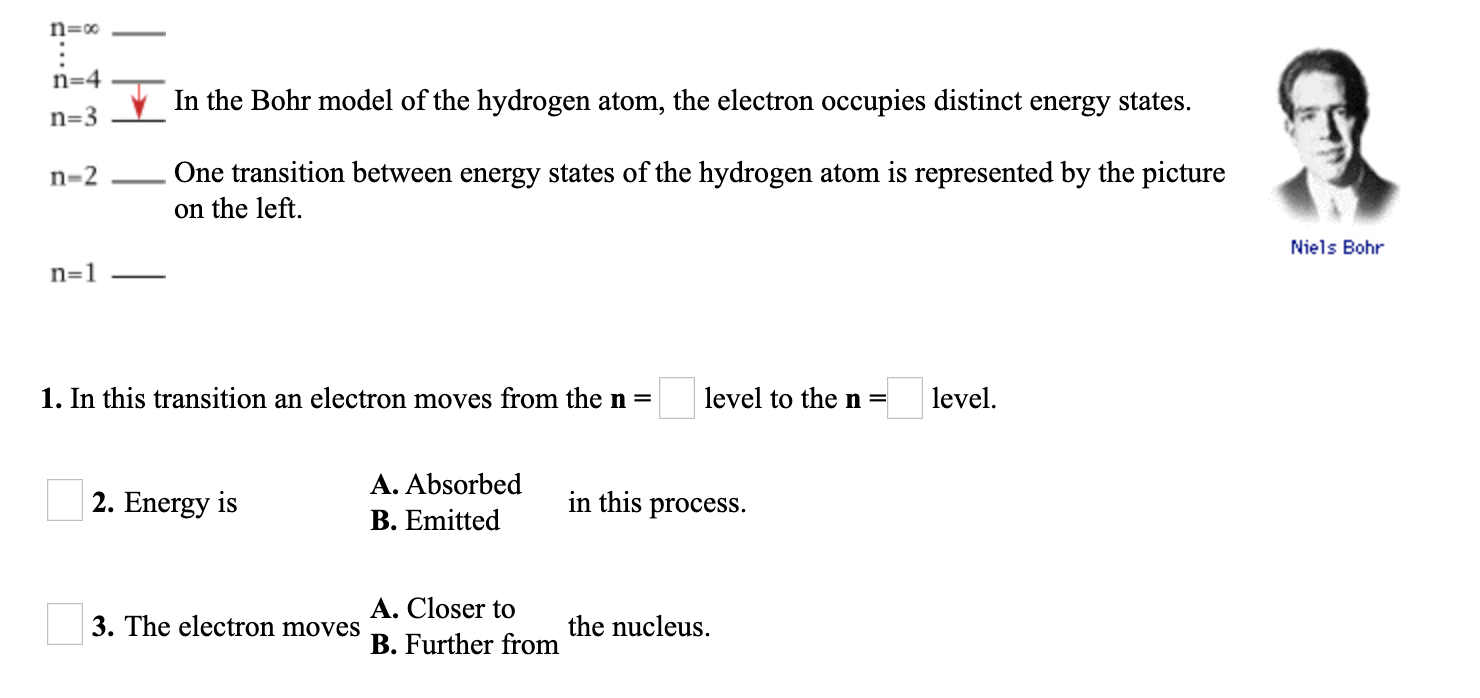
Transcribed Image Text:n=4
V In the Bohr model of the hydrogen atom, the electron occupies distinct energy states.
n=3
One transition between energy states of the hydrogen atom is represented by the picture
on the left.
n-2
Niels Bohr
n=1
1. In this transition an electron moves from the n =
level to the n =
level.
A. Absorbed
2. Energy is
in this process.
B. Emitted
A. Closer to
3. The electron moves
the nucleus.
B. Further from
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
