NADH can act as an inhibitor of isocitrate dehydrogenase and a-ketoglutarate dehydrogenase. This is an example of:
NADH can act as an inhibitor of isocitrate dehydrogenase and a-ketoglutarate dehydrogenase. This is an example of:
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
I need help anwsering all the following questions thankyou!

Transcribed Image Text:QUESTION 11
NADH can act as an inhibitor of isocitrate dehydrogenase and a-ketoglutarate dehydrogenase. This is an
example of:
Negative allosteric regulation
Feedback inhibition
Feed-forward activation
Product inhibition
QUESTION 12
When the flux of the citric acid cycle is low, Acetyl-CoA accumulates. The high levels of Acetyl-CoA activate
pyruvate carboxylase, which synthesizes oxaloacetate. This is an example of:
a fermentation reaction
substrate level phosphorylation
an anaplerotic reaction
a cataplerotic reaction
QUESTION 13
How does insulin influence glucose metabolism?
O Leads to dephosphorylation of the PFK2/FBPase2 enzyme
Activates to phosphorolysis of glycogen
Leads to dephosphorylation of PFK1
Leads to activation of FBPase1
QUESTION 14
31. Which of these substances from the Electron Transport Chain has the highest standard reduction potential (e°)?
O2
QH2
NADH
cytochrome c
QUESTION 15
Upon addition of Molecule X to actively respiring mitochondria, the QH2/Q ratio increased (higher levels of QH2
compared to Q). Based on this observation: which component of the Electron Transport Chain is becoming
inhibited by Molecule X?
O Complex I
Complex II
Complex IV
Complex III
0000
000 o
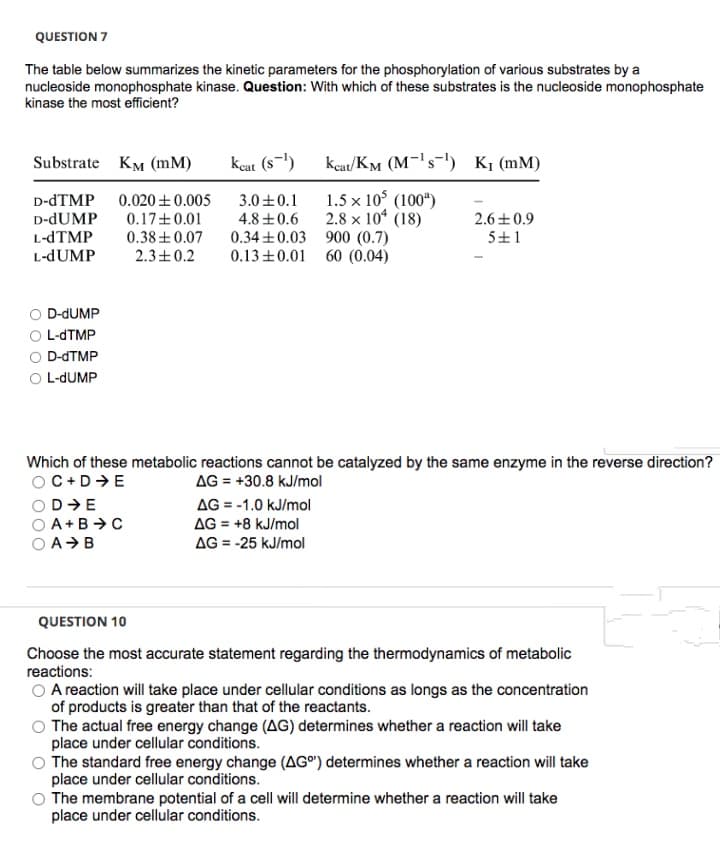
Transcribed Image Text:QUESTION 7
The table below summarizes the kinetic parameters for the phosphorylation of various substrates by a
nucleoside monophosphate kinase. Question: With which of these substrates is the nucleoside monophosphate
kinase the most efficient?
Substrate KM (mM)
kcat (s-)
keat/KM (M-'s-') K¡ (mM)
D-dTMP
D-dUMP
L-dTMP
L-dUMP
1.5 x 10° (100“)
2.8 x 10* (18)
0.34 +0.03 900 (0.7)
0.13+0.01 60 (0.04)
0.020 +0.005
3.0+0.1
2.6+0.9
0.17+0.01
0.38 +0.07
2.3+0.2
4.8 +0.6
5+1
O D-DUMP
L-DTMP
D-dTMP
O L-DUMP
Which of these metabolic reactions cannot be catalyzed by the same enzyme in the reverse direction?
OC+D+E
AG = +30.8 kJ/mol
OD E
A+B>C
AG = -1.0 kJ/mol
AG = +8 kJ/mol
AG = -25 kJ/mol
%3D
O AB
QUESTION 10
Choose the most accurate statement regarding the thermodynamics of metabolic
reactions:
O A reaction will take place under cellular conditions as longs as the concentration
of products is greater than that of the reactants.
O The actual free energy change (AG) determines whether a reaction will take
place under cellular conditions.
The standard free energy change (AG") determines whether a reaction will take
place under cellular conditions.
O The membrane potential of a cell will determine whether a reaction will take
place under cellular conditions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON