• Normal boiling point of carbon tetrachloride is 76.8 °C a. Calculate the boiling point of a solution made by mixing 25.56 grams of solid iodine with 250 ml carbon tetrachloride. (Show all work) b. Calculate the boiling point of a solution made by mixing 25.56 grams of solid naphthalene (C10H8) with 250 ml carbon tetrachloride. (Show all work). a. dasie Cly I S14 glml cely mass 14260m InL 4.954(/m l 25.06g l2) 253. flgimal 1.396kg 59 U59 76.3 79 1'C Same a1 above 4.95x 255 121 174 2.니43 14glmole. 741'4 2.943= 9 19 c. In referring back to the introduction, does this calculation support or refute the statement "Some properties of the solvent are changed only by the number of solute particles present, without regard to the particular chemical nature of the solute"? Explain.
• Normal boiling point of carbon tetrachloride is 76.8 °C a. Calculate the boiling point of a solution made by mixing 25.56 grams of solid iodine with 250 ml carbon tetrachloride. (Show all work) b. Calculate the boiling point of a solution made by mixing 25.56 grams of solid naphthalene (C10H8) with 250 ml carbon tetrachloride. (Show all work). a. dasie Cly I S14 glml cely mass 14260m InL 4.954(/m l 25.06g l2) 253. flgimal 1.396kg 59 U59 76.3 79 1'C Same a1 above 4.95x 255 121 174 2.니43 14glmole. 741'4 2.943= 9 19 c. In referring back to the introduction, does this calculation support or refute the statement "Some properties of the solvent are changed only by the number of solute particles present, without regard to the particular chemical nature of the solute"? Explain.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Part c
Transcribed Image Text:Course: CHM.Q14001-22SP Ge X 4 Shared with me - Google Drive x E Exp. 7 A&D - Google Docs
G . In referring back to th
em/document/d/Kg1dODP5kkUosmV5gK5IMtKIT4hK2CJglLO3nZOtGnU/edit
ed From Fir. M HBO Max
at Tools Add-ons Help
Last edit was
Unsupported image type.
Dismiss
rmal text
+ BIU
E= E -E - E E X
Arial
11
1
.| I2 I I3 I · I4 II I5 III I 6 II
7
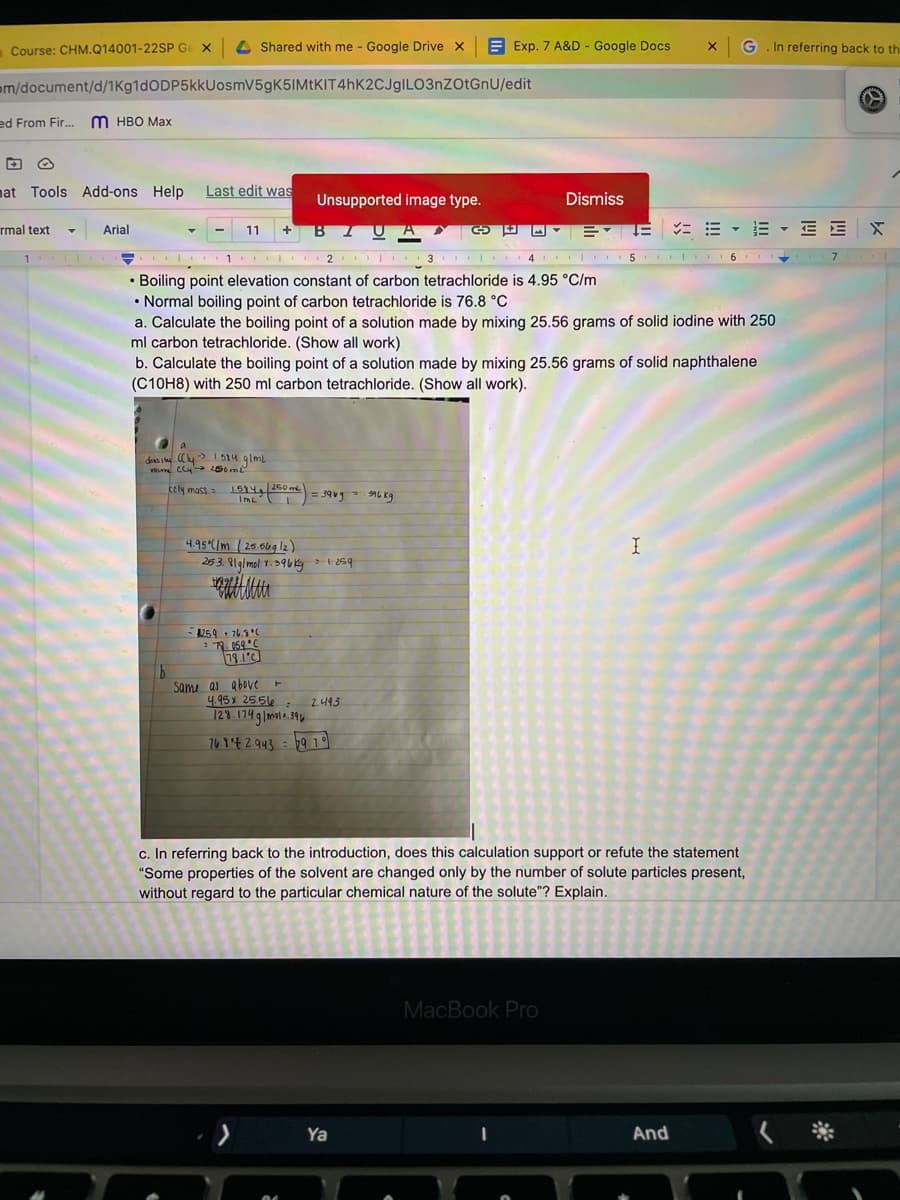
Boiling point elevation constant of carbon tetrachloride is 4.95 °C/m
• Normal boiling point of carbon tetrachloride is 76.8 °C
a. Calculate the boiling point of a solution made by mixing 25.56 grams of solid iodine with 250
ml carbon tetrachloride. (Show all work)
b. Calculate the boiling point of a solution made by mixing 25.56 grams of solid naphthalene
(C10H8) with 250 ml carbon tetrachloride. (Show all work).
a.
das i Cy 1514 glml
cely mass
15744 260 me
IML
%3D
4.95 C/m ( 25.6ug l2)
253. 8lg/mol Y.596kg 1259
259• 76.5
79.1'C
b.
Same as above -
4.95 x 25.5
129.174glmols.39
e : 2.493
76.9'42.943 =b9 19
c. In referring back to the introduction, does this calculation support or refute the statement
"Some properties of the solvent are changed only by the number of solute particles present,
without regard to the particular chemical nature of the solute"? Explain.
MacBook Pro
Ya
And
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY