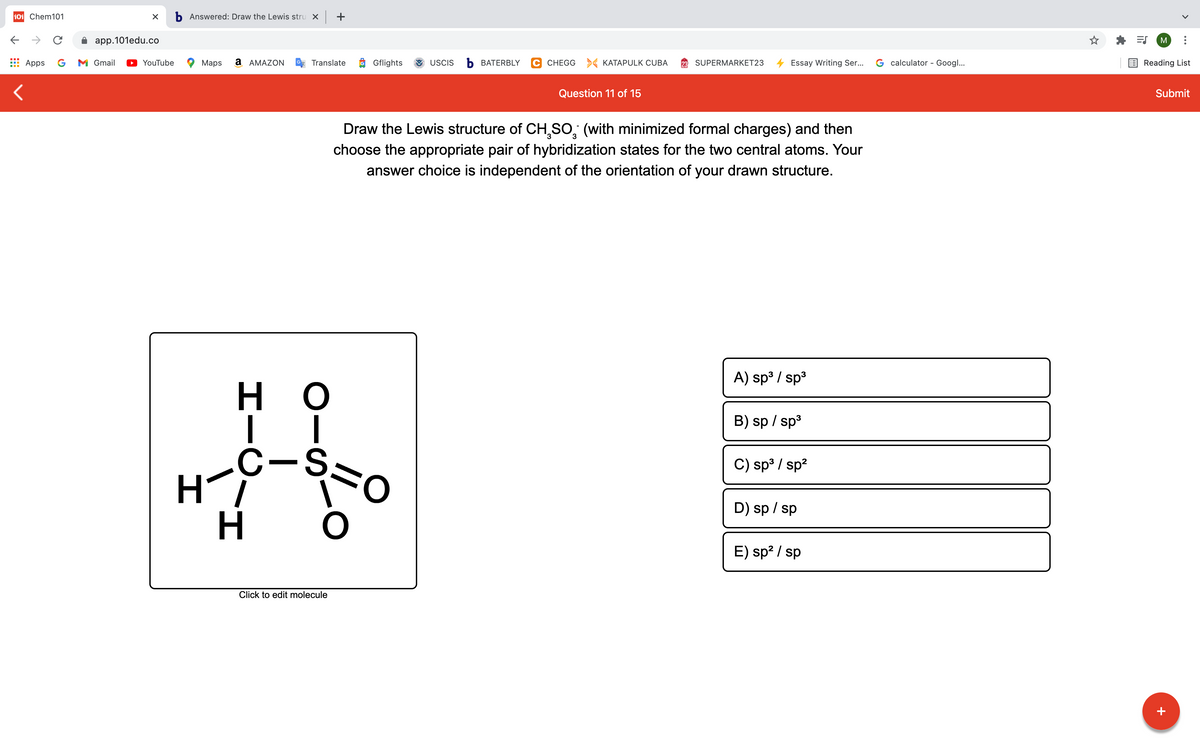
Draw the Lewis structure of CH,So, (with minimized formal charges) and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure. A) sp / sp но B) sp / sp C-S. C) sp / sp? D) sp / sp H E) sp? / sp Click to edit molecule
Draw the Lewis structure of CH,So, (with minimized formal charges) and then choose the appropriate pair of hybridization states for the two central atoms. Your answer choice is independent of the orientation of your drawn structure. A) sp / sp но B) sp / sp C-S. C) sp / sp? D) sp / sp H E) sp? / sp Click to edit molecule
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.43QE: For each of the following molecules, complete the Lewis structure and use the VSEPR model to...
Related questions
Question
Transcribed Image Text:101 Chem101
b Answered: Draw the Lewis stru x +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
a AMAZON
Translate
O Gflights
USCIS
Ь ВАТERBLY
C CHEGG KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
Reading List
Question 11 of 15
Submit
Draw the Lewis structure of CH,SO, (with minimized formal charges) and then
choose the appropriate pair of hybridization states for the two central atoms. Your
answer choice is independent of the orientation of your drawn structure.
A) sp3 / sp3
H.
B) sp / sp3
C-,
Hi
కొం
C) sp³ / sp?
D) sp / sp
E) sp? / sp
Click to edit molecule
+
エー
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning