o a 100-mL volumetric flask. The 20.0-mL aliquot was treated with 10.0 mL of 10.0 M NAOH plus enough Nal to complex the Hg cat- alyst from the digestion and diluted to 100.0 mL. When measured with the ammonia electrode, this solution gave a reading of 339.3 mV. Calculate the wt% nitrogen in the food sample.
o a 100-mL volumetric flask. The 20.0-mL aliquot was treated with 10.0 mL of 10.0 M NAOH plus enough Nal to complex the Hg cat- alyst from the digestion and diluted to 100.0 mL. When measured with the ammonia electrode, this solution gave a reading of 339.3 mV. Calculate the wt% nitrogen in the food sample.
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.17QAP
Related questions
Question
100%
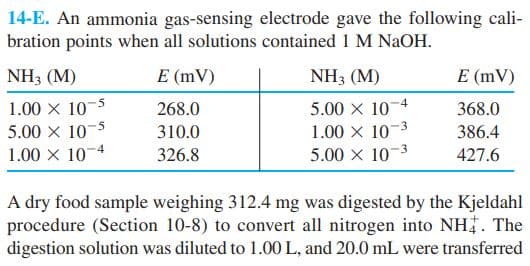
Transcribed Image Text:14-E. An ammonia gas-sensing electrode gave the following cali-
bration points when all solutions contained 1 M NaOH.
NH3 (M)
E (mV)
NH3 (M)
E (mV)
1.00 x 10-5
5.00 x 10
1.00 x 10
5.00 x 103
268.0
368.0
5
-3
5.00 X 10
310.0
386.4
1.00 X 10-4
326.8
427.6
A dry food sample weighing 312.4 mg was digested by the Kjeldahl
procedure (Section 10-8) to convert all nitrogen into NH. The
digestion solution was diluted to 1.00 L, and 20.0 mL were transferred

Transcribed Image Text:to a 100-mL volumetric flask. The 20.0-mL aliquot was treated with
10.0 mL of 10.0M NaOH plus enough Nal to complex the Hg cat-
alyst from the digestion and diluted to 100.0 mL. When measured
with the ammonia electrode, this solution gave a reading of 339.3 mV.
Calculate the wt% nitrogen in the food sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 1 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning