A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken. Ammeter 6-12 volts Switch Carbon Electrodes Test Solution Conductivity Apparatus Solution Reading 0.1 M H2SO4 150 mA 0.1 M Ba(OH)2 150 mA To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken. Ammeter 6-12 volts Switch Carbon Electrodes Test Solution Conductivity Apparatus Solution Reading 0.1 M H2SO4 150 mA 0.1 M Ba(OH)2 150 mA To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
Explain the data.
Is there any evidence that a reaction has occurred?
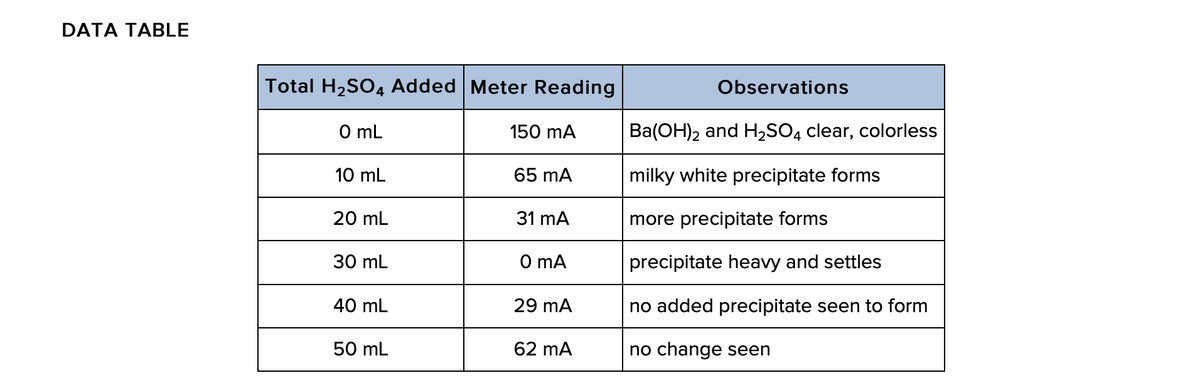
Transcribed Image Text:DATA TABLE
Total H2SO4 Added Meter Reading
Observations
O mL
150 mA
Ba(OH)2 and H2SO4 clear, colorless
10 mL
65 mA
milky white precipitate forms
20 mL
31 mA
more precipitate forms
30 mL
O mA
precipitate heavy and settles
40 mL
29 mA
no added precipitate seen to form
50 mL
62 mA
no change seen
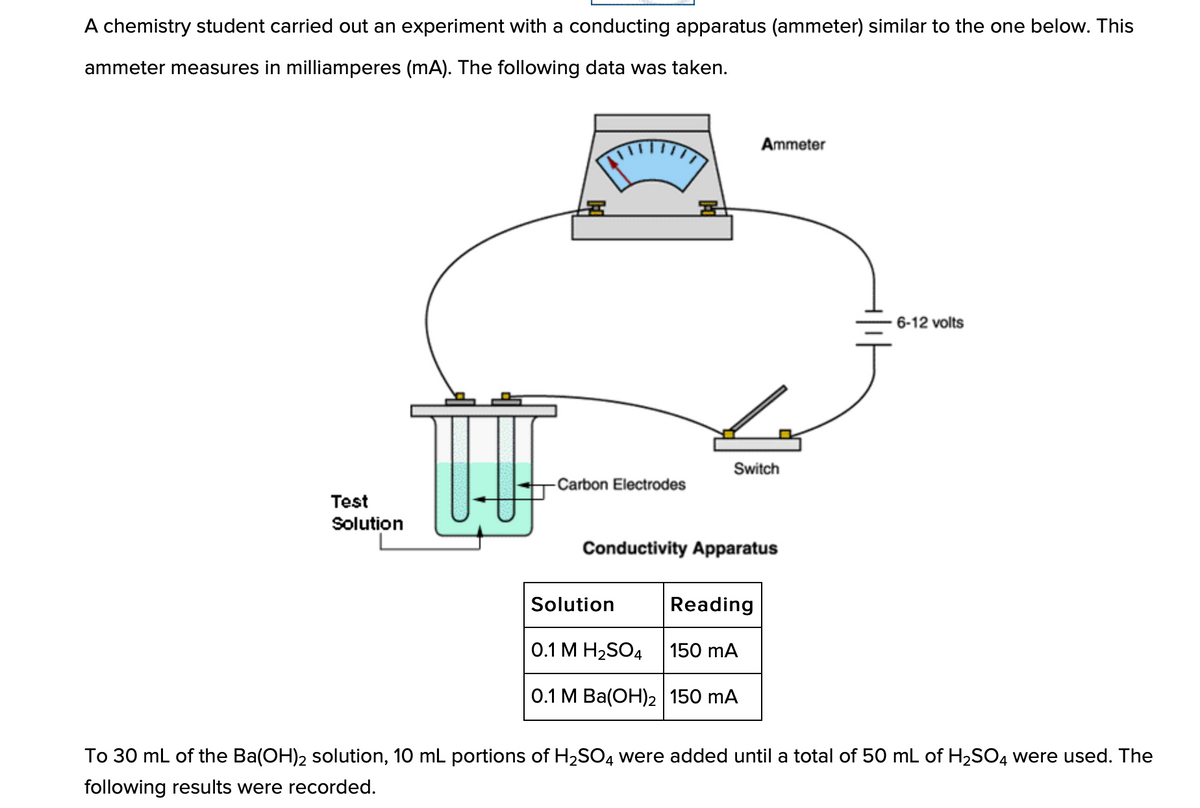
Transcribed Image Text:A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This
ammeter measures in milliamperes (mA). The following data was taken.
Ammeter
6-12 volts
Switch
-Carbon Electrodes
Test
Solution
Conductivity Apparatus
Solution
Reading
0.1 М Н2SO4
150 mA
О.1 М Ba(ОН)2 | 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The
following results were recorded.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

