: Он O : : OH -H- C-CH3 + H CH3 CH,-C-CH3 R CH,-C-CH3 step 2 step 3 : NH2 step 1 NH3 step 4 CH,--CH, (NH, -H,0 T V CH,-C-CH3 || NH step 6 step 5 i) Write the missing structures (0, P, Q and R) in the above reaction scheme. ii) Name the class of compound, molecule S belongs to? iii) This is an acid catalyzed reaction. What does this statement mean? iv) Draw the arrow diagrams for steps 1 and 4 to show how intermediate O and Q are formed? v) Write the equation for this step ONLY. vi) Step 4 allows a generation of a good leaving group in intermediate Q. Name the leaving group in this step. Steps 3 and 6 involve loss of a proton from the intermediate ions. Why is this step necessary?
: Он O : : OH -H- C-CH3 + H CH3 CH,-C-CH3 R CH,-C-CH3 step 2 step 3 : NH2 step 1 NH3 step 4 CH,--CH, (NH, -H,0 T V CH,-C-CH3 || NH step 6 step 5 i) Write the missing structures (0, P, Q and R) in the above reaction scheme. ii) Name the class of compound, molecule S belongs to? iii) This is an acid catalyzed reaction. What does this statement mean? iv) Draw the arrow diagrams for steps 1 and 4 to show how intermediate O and Q are formed? v) Write the equation for this step ONLY. vi) Step 4 allows a generation of a good leaving group in intermediate Q. Name the leaving group in this step. Steps 3 and 6 involve loss of a proton from the intermediate ions. Why is this step necessary?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 31E
Related questions
Question
Do the highlights
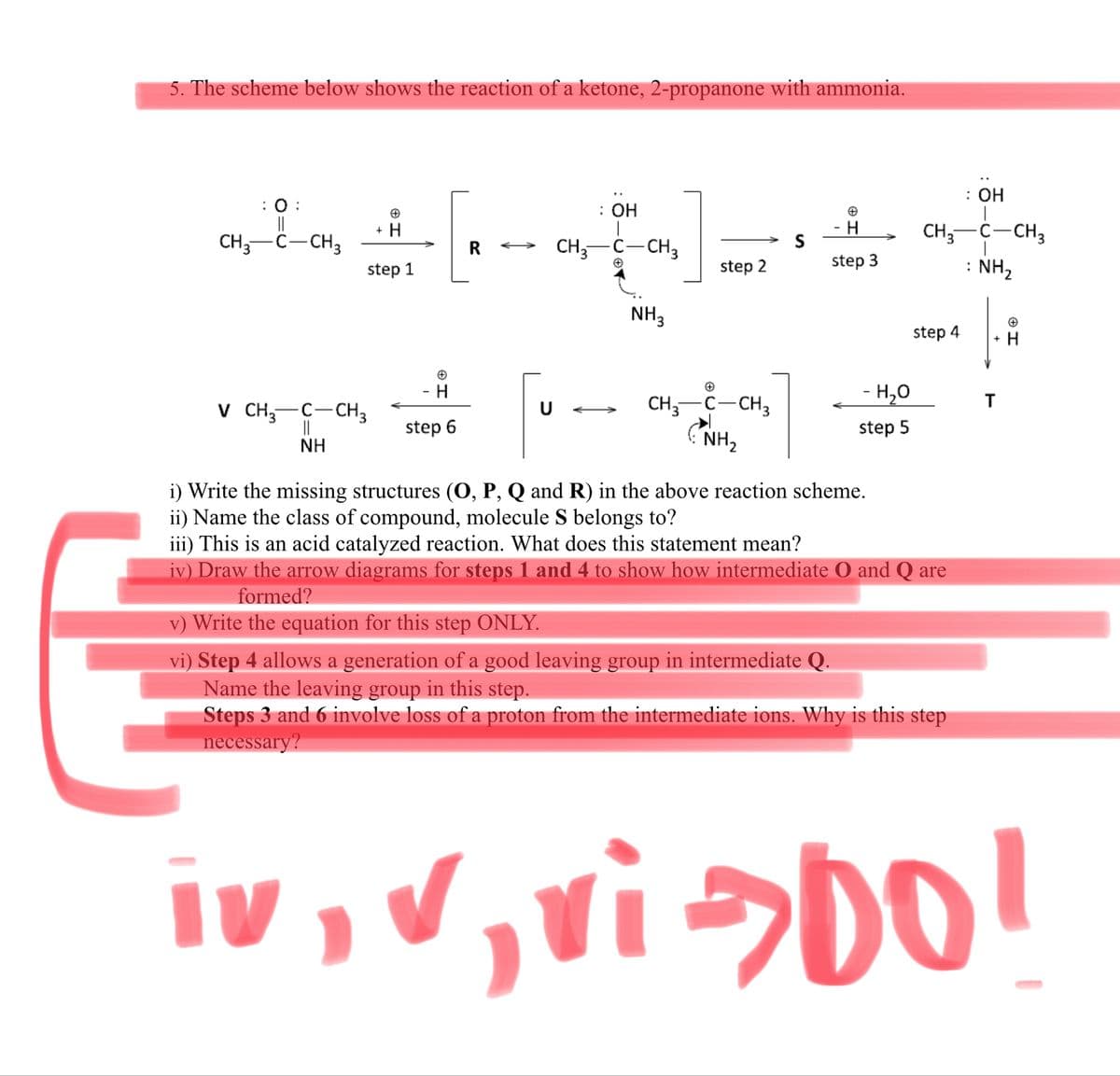
Transcribed Image Text:5. The scheme below shows the reaction of a ketone, 2-propanone with ammonia.
: ОН
: :
||
CH,-C-CH3
: ОН
+ H
H
CH;-C-CH3
: NH2
S
R
CH,-C-CH,
step 1
step 2
step 3
NH3
step 4
+ H
- H
CH; C-CH3
- H,0
T
V CH;-C-CH3
||
>
step 6
step 5
NH
NH,
i) Write the missing structures (O, P, Q and R) in the above reaction scheme.
ii) Name the class of compound, molecule S belongs to?
iii) This is an acid catalyzed reaction. What does this statement mean?
iv) Draw the arrow diagrams for steps 1 and 4 to show how intermediate O and Q are
formed?
v) Write the equation for this step ONLY.
vi) Step 4 allows a generation of a good leaving group in intermediate Q.
Name the leaving group in this step.
Steps 3 and 6 involve loss of a proton from the intermediate ions. Why is this step
necessary?
iv, V,vi>DO!
Expert Solution
Step 1
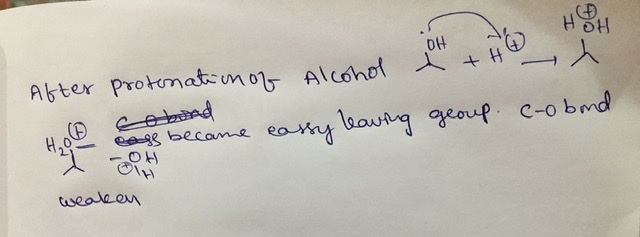
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
