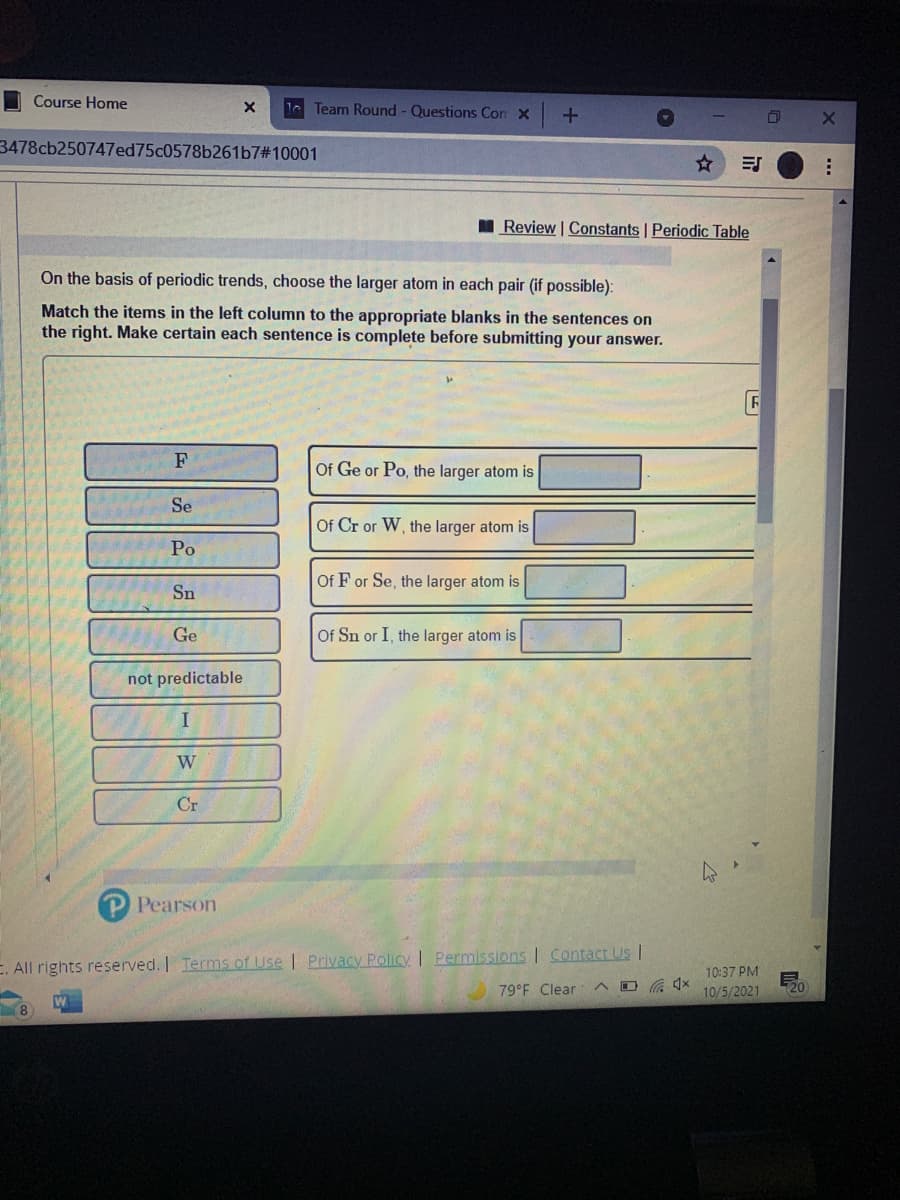
On the basis of periodic trends, choose the larger atom in each pair (if possible): Match the items in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. F Of Ge or Po, the larger atom is Se Of Cr or W, the larger atom is Ро Of F or Se, the larger atom is Sn Ge Of Sn or I, the larger atom is not predictable W Cr
On the basis of periodic trends, choose the larger atom in each pair (if possible): Match the items in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. F Of Ge or Po, the larger atom is Se Of Cr or W, the larger atom is Ро Of F or Se, the larger atom is Sn Ge Of Sn or I, the larger atom is not predictable W Cr
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 12ALQ
Related questions
Question
Transcribed Image Text:Course Home
le Team Round - Questions Con X
3478cb250747ed75c0578b261b7#10001
I Review | Constants | Periodic Table
On the basis of periodic trends, choose the larger atom in each pair (if possible):
Match the items in the left column to the appropriate blanks in the sentences on
the right. Make certain each sentence is complete before submitting your answer.
F
Of Ge or Po, the larger atom is
Se
Of Cr or W, the larger atom is
Po
Of F or Se, the larger atom is
Sn
Ge
Of Sn or I, the larger atom is
not predictable
I
W
Cr
Pearson
E. All rights reserved. I Terms of Use | Privacy Policy I Permissions I Contact Us I
10:37 PM
O G 4x 10/5/2021
79°F Clear
20
...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning