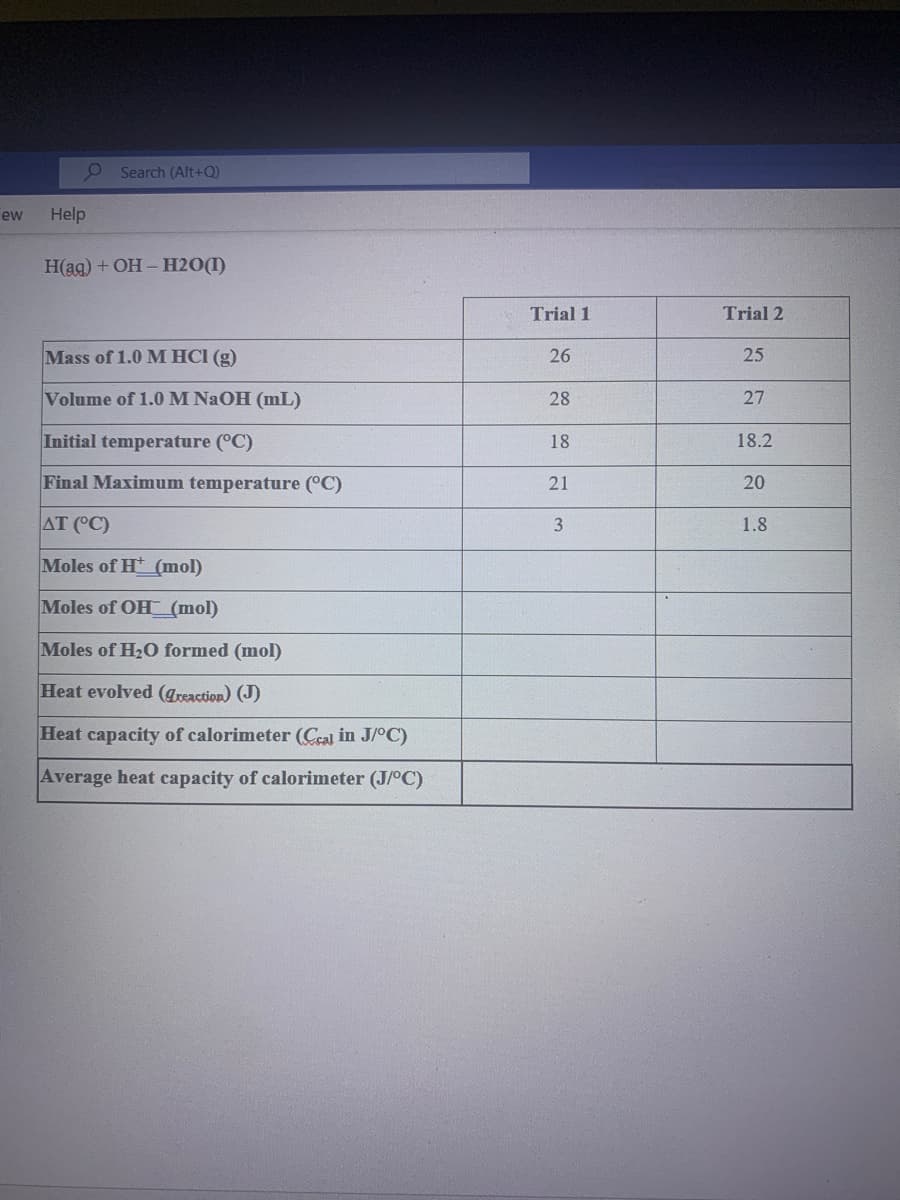
P Search (Alt+Q) ew Help H(ag) + OH – H2O(I) Trial 1 Trial 2 Mass of 1.0 M HCI (g) 26 25 Volume of 1.0M NAOH (mL) 28 27 Initial temperature (°C) 18 18.2 Final Maximum temperature (°C) 21 20 AT (°C) 1.8 Moles of H (mol) Moles of OH (mol) Moles of H2O formed (mol) Heat evolved (areaction) (J) Heat capacity of calorimeter (Ceal in J/°C) Average heat capacity of calorimeter (J/°C) 3.
P Search (Alt+Q) ew Help H(ag) + OH – H2O(I) Trial 1 Trial 2 Mass of 1.0 M HCI (g) 26 25 Volume of 1.0M NAOH (mL) 28 27 Initial temperature (°C) 18 18.2 Final Maximum temperature (°C) 21 20 AT (°C) 1.8 Moles of H (mol) Moles of OH (mol) Moles of H2O formed (mol) Heat evolved (areaction) (J) Heat capacity of calorimeter (Ceal in J/°C) Average heat capacity of calorimeter (J/°C) 3.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.27QAP
Related questions
Question
Please show all the steps and how to find the moles of each one by showing the steps not only the answer. Thanks
Transcribed Image Text:9 Search (Alt+Q)
ew
Help
Нaa) + ОН - Н20()
Trial 1
Trial 2
Mass of 1.0 M HCI (g)
26
25
Volume of 1.0M NAOH (mL)
28
27
Initial temperature (°C)
18
18.2
Final Maximum temperature (°C)
21
20
AT (°C)
3
1.8
Moles of H (mol)
Moles of OH (mol)
Moles of H20 formed (mol)
Heat evolved (Treaction) (J)
Heat capacity of calorimeter (Ceal in J/°C)
Average heat capacity of calorimeter (J/°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



