Part A Calculate the number of moles of are no molecules of B at time Review 1 Constants l Periodic Table B at 10 min, assuming that there Consider the following hypothetical aqueous reaction: A(a)+B(aq). A flask is charged with 0.065 mol of A in a total volume of 100.0 mL. The following data are collected zero. Express your answer using two significant figures. Time (min) 0 10 20 30 40 Moles of A 0.065 0.051 0.042 0.036 0.031 ΑΣφ nB Submit Request Answer Part B Calculate the number of moles of B at 20 min, assuming that there are no molecules of B at time zero. Express your answer using two significant figures. ΑΣφ 72B P Pearson 12:02 a, m 19 02/2019 7
Part A Calculate the number of moles of are no molecules of B at time Review 1 Constants l Periodic Table B at 10 min, assuming that there Consider the following hypothetical aqueous reaction: A(a)+B(aq). A flask is charged with 0.065 mol of A in a total volume of 100.0 mL. The following data are collected zero. Express your answer using two significant figures. Time (min) 0 10 20 30 40 Moles of A 0.065 0.051 0.042 0.036 0.031 ΑΣφ nB Submit Request Answer Part B Calculate the number of moles of B at 20 min, assuming that there are no molecules of B at time zero. Express your answer using two significant figures. ΑΣφ 72B P Pearson 12:02 a, m 19 02/2019 7
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 129QRT
Related questions
Question
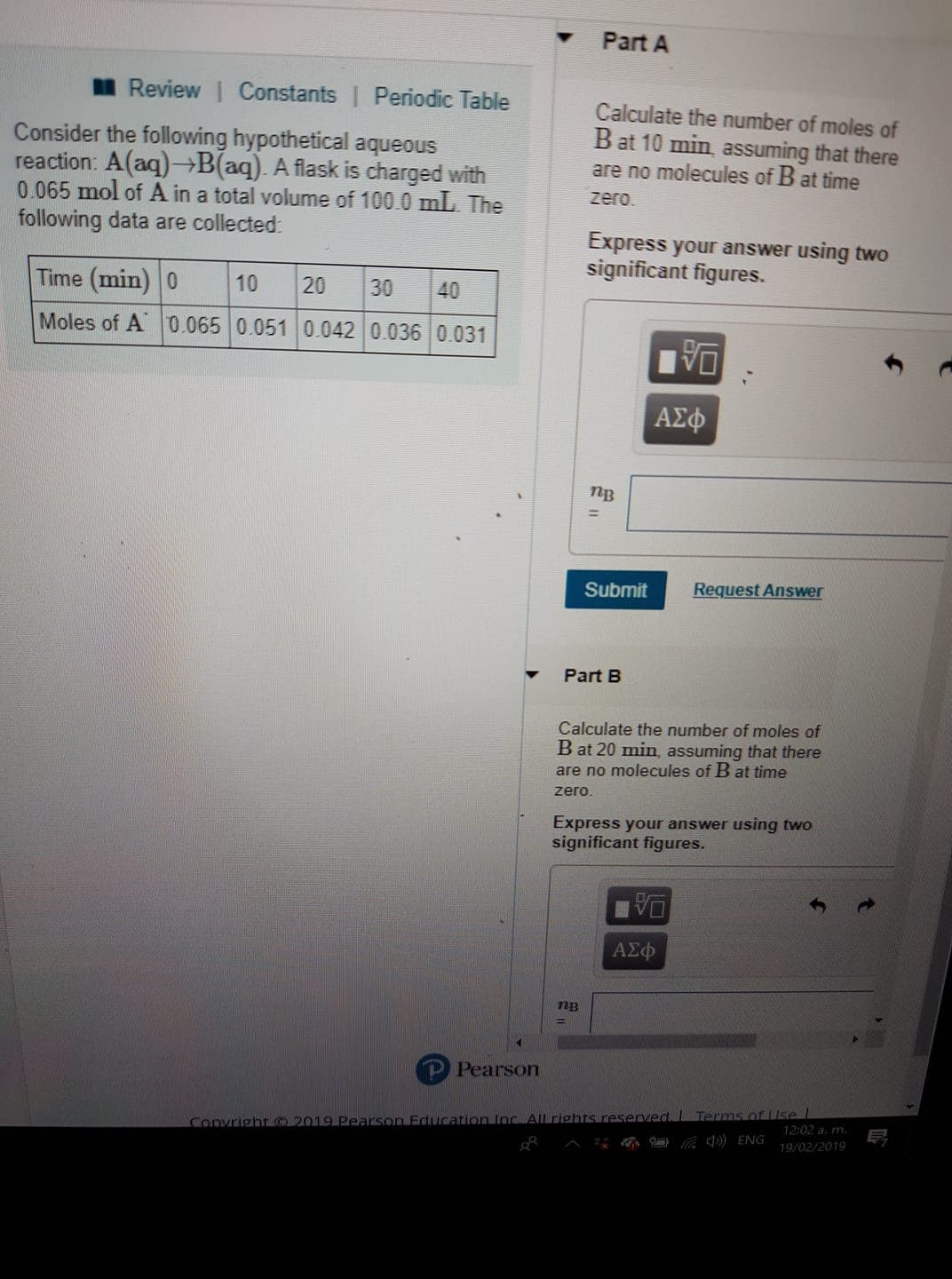
Transcribed Image Text:Part A
Calculate the number of moles of
are no molecules of B at time
Review 1 Constants l Periodic Table
B at 10 min, assuming that there
Consider the following hypothetical aqueous
reaction: A(a)+B(aq). A flask is charged with
0.065 mol of A in a total volume of 100.0 mL. The
following data are collected
zero.
Express your answer using two
significant figures.
Time (min) 0 10 20 30 40
Moles of A 0.065 0.051 0.042 0.036 0.031
ΑΣφ
nB
Submit
Request Answer
Part B
Calculate the number of moles of
B at 20 min, assuming that there
are no molecules of B at time
zero.
Express your answer using two
significant figures.
ΑΣφ
72B
P Pearson
12:02 a, m
19 02/2019
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 4 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax