Lab Sec. Name Desk No. A. Standardized HCI Solution Molar mass Naaco3 Hao =124.0glmol Calculate the mass of sodium carbonate sample required in Part A.3. eub b Iavongge Trial 1 Trial 2 Trial 3 Trial 4 1. Tared mass of Na,CO, (g) 0.305 O.373 0-36 0.304 2. Moles of Na,CO, (mol) 0.00278 0.00301 0.00290 0.00245 3. Buret reading, initial (mL) 0.) 3.29 4. Buret reading, final (mL) 24.7 27.93 23.99 20.7 5. Volume of HCl added (mL) 23.39 24.64 Tonnion noo 000ssla 0.ml002 0.Oosgo 0.000490 24.660 20.5 o and 6. Moles of HCI added (mol) 7. Molar concentration of HCI (mo/L) 0,244 0.248 o.239 8. Average molar concentration of HCl (mo/L) 0.239M B. Preparation of Borax Solution Sample number 5.00 5.00 5.00 5.00 5.00 5.00 1. Volume of sample (mL) 48 40 32 2s.1 4.5 2. Temperature of sample (°C) C. Analysis of Borax Test Solutions 3.29 0.6 A.2 0.8 0.48 1. Buret reading, initial (mL) 27.93 24.7 23.99 20.7 교니.S / 21.62 2. Buret reading, final (mL) 17.662 13.01 9.1 3.06 25.2 3. Volume of HCl added (mL)
Lab Sec. Name Desk No. A. Standardized HCI Solution Molar mass Naaco3 Hao =124.0glmol Calculate the mass of sodium carbonate sample required in Part A.3. eub b Iavongge Trial 1 Trial 2 Trial 3 Trial 4 1. Tared mass of Na,CO, (g) 0.305 O.373 0-36 0.304 2. Moles of Na,CO, (mol) 0.00278 0.00301 0.00290 0.00245 3. Buret reading, initial (mL) 0.) 3.29 4. Buret reading, final (mL) 24.7 27.93 23.99 20.7 5. Volume of HCl added (mL) 23.39 24.64 Tonnion noo 000ssla 0.ml002 0.Oosgo 0.000490 24.660 20.5 o and 6. Moles of HCI added (mol) 7. Molar concentration of HCI (mo/L) 0,244 0.248 o.239 8. Average molar concentration of HCl (mo/L) 0.239M B. Preparation of Borax Solution Sample number 5.00 5.00 5.00 5.00 5.00 5.00 1. Volume of sample (mL) 48 40 32 2s.1 4.5 2. Temperature of sample (°C) C. Analysis of Borax Test Solutions 3.29 0.6 A.2 0.8 0.48 1. Buret reading, initial (mL) 27.93 24.7 23.99 20.7 교니.S / 21.62 2. Buret reading, final (mL) 17.662 13.01 9.1 3.06 25.2 3. Volume of HCl added (mL)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.22QAP
Related questions
Question
Please help me calculate for A
Thank you
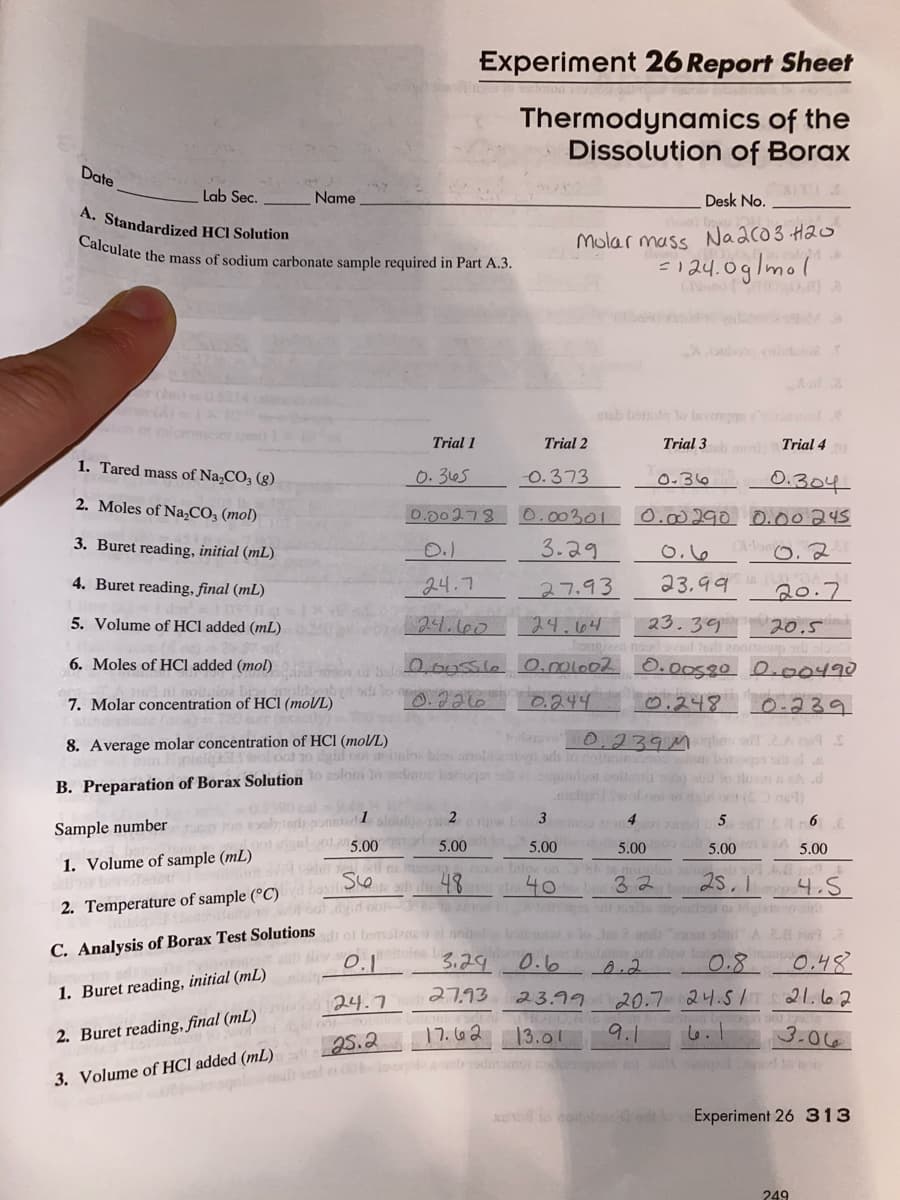
Transcribed Image Text:Calculate the mass of sodium carbonate sample required in Part A.3.
Experiment 26 Report Sheet
Thermodynamics of the
Dissolution of Borax
Date
Lab Sec.
Name
Desk No.
A. Standardized HCI Solution
Molar massNaaco3 Hao
=124.0g/mol
ctab boole lo lavongge
Trial 1
Trial 2
Trial 3
Trial 4
1. Tared mass of Na,CO3 (g).
0. 365
O.373
0.304
0.36
2. Moles of Na,CO3 (mol)
0.00278
0.00301
0.00290 O.00245
3. Buret reading, initial (mL)
3.29
0.16
4. Buret reading, final (mL)
24.7
27.93
23,99
20.7
23.39
lo/sd hdi ano p li
O.00580 0.00490
5. Volume of HCl added (mL)
24.660
24.64
20.5
bone
6. Moles of HCl added (mol)
7. Molar concentration of HCI (mol/L)
0.244
0.248 0-239
8. Average molar concentration of HCl (mol/L)
ool 20 dgid no uni
ove
adh io nothins on
0.239Mogbn
o banuqon
B. Preparation of Borax Solutionto aslom In
1
2 rw Lo 3
Sample number
5.00
5.00
5.00
5.00
5.00 A 5.00
1. Volume of sample (mL)
40
32
25.1
4.5
2. Temperature of sample (°C)
28 h2
C. Analysis of Borax Test Solutions o bemalzg
3.29
0.6
0.8
0.48
1. Buret reading, initial (mL)
27.93
21.62
20.7 24.51
9.1
24.7
23.99
2. Buret reading, final (mL)
2רו
13.01
6.1
3.06
25.2
3. Volume of HCl added (mL)
xevod io noi dto Experiment 26 313
249

Transcribed Image Text:In order to calculate the mass of sodium carbonate
asked for in the first question at the top of the report
page-
estimate 0.25 M x 0.020 L = #### x 1 CO32/2 H+ =
##### x 124.01 g/ mol
We are using the monohydrated version of sodium
carbonate for this lab Na2CO3*H2O
Use next set of data to fill in temps and volumes of
HCL for parts B & C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
