P17D.3 The rate constant for the gas-phase reaction of ethene and hydrogen, C,H,(g)+H,(g)→C,H,(g), was measured at different temperatures. Use the following values to calculate the Arrhenius parameters. T/K 1000 1200 1400 1600 k/(dm' mol's) 8.35 x 10-10 3.08 x 10 4.06 x 107 2.80 x 106
P17D.3 The rate constant for the gas-phase reaction of ethene and hydrogen, C,H,(g)+H,(g)→C,H,(g), was measured at different temperatures. Use the following values to calculate the Arrhenius parameters. T/K 1000 1200 1400 1600 k/(dm' mol's) 8.35 x 10-10 3.08 x 10 4.06 x 107 2.80 x 106
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.60E
Related questions
Question
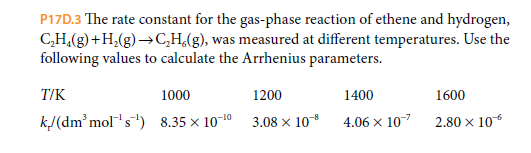
Transcribed Image Text:P17D.3 The rate constant for the gas-phase reaction of ethene and hydrogen,
C,H,(g)+H,(g)→C,H,(g), was measured at different temperatures. Use the
following values to calculate the Arrhenius parameters.
T/K
1000
1200
1400
1600
k/(dm' mol's) 8.35 x 10-10
3.08 x 10
4.06 x 107
2.80 x 106
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning